ALL ABOUT COLORRock and Minerals of Color, Color Pigments, Color Elements, Color Ores, Artist's Real Color Wheel, Ore's Color Reactions, Crystal Color Wheel, Prism Colors, Color Theory, RainbowsBiography. 4 Videos of Location Painting. Household Cylopedia, 1881, colors of paints & inks ARTISTS, dated history, pigments, color theory, techniques. LOCATION, all media, human proportions, perspective, modern techniques. COLOR, B.C. to A/D pigments, RCW, crystal chart, elements, minerals, ores, rainbows, prisms. HISTORY, comparative advances in art, European and Asian Cultures, 8000 B.C. to 1912 MEDIA, supports, oil, acrylic, water color, wax, cera colla, casein, fresco. Coloring Book, pattern outlines, aerial perspective palette. B/C MINING
B/C PALETTE
CRYSTAL ELEMENT-TO-COLOR CHART
TWELVE STANDARD (Real Color Wheel) COLORS IN MINERAL COMPOUNDS 89
B/C PALETTE 97
THE REAL COLOR WHEEL (RCW) 221
MOHS SCALE OF HARDNESS
BASALT Basalt is the most common of the earth's volcanic rocks, its crystals are continuous and elongated. Basalt magma is sometimes as hard as glass, but rarely. SYENITE Syenite forms in silicate-less magma, consisting typically of feldspar and hornblende. QUARTZ Quartz is the most common crystal mineral, oxygen and silicon, it has the hardness of H7. Quartz is found in ore mineral veins and needs a hollow space to form, As rocks and veins weather, quartz is freed of its matrix and breaks loose to form sand. Cemented sand is sandstone. Intruding magma going through sandstone will again form ore and quartz. Prospectors say gold is found near quartz. GRANITE Granite is also igneous, fire intrusive. It's composed of quartz and feldspar. Granite magma intrudes limestone or dolomite and forms marble. Marble is heat and calcium of limestone, plus calcium magnesium of dolomite, plus silica. Veins or lodes of ore in marble include, tin, copper, uranium, iron, zinc and lead. Marble is metamorphic limestone. Granite intrudes with its hottest leading edge forming cassiterite, tin. The copper sulfides form second, chalcopyrite is the primary copper ore formed in quartz. Third would be cobalt, nickel and arsenic. Than the zinc-lead zone, with zinc sulfide as sphalerite ore, lead sulfide as galena ore, than silver. Finally, the iron rich zone with iron carbonate as siderite ore, the dominate mineral of the world. FELDSPAR Feldspar is common in granite magma intruding on sandstone sedimentary rock, it hardens into a crystal softer then quartz. Feldspar can be morphasized with pressure to a harder crystal like tourmaline or topaz. Feldspar is the second most common mixed mineral silicate crystal, made from potassium, sodium and calcium, an alumino-silicate. It contains aluminum in the ore, bauxite, clay and rock. Clay is hydrated silicates of aluminum. Potash feldspar is microdine, add iron and it takes on red to green colors. Green amazonite was mined and used as a pigment on murals in 1300 B/C, in Egypt. Plageoclase feldspar is sodium and calcium.
KAOLINITE
Kaolinite, is clay and chalk after feldspar. Carbonic acid, is present in rainwater or vapors, this pheudomorphs the kaolinite from feldspar. Porcelain is made from kaolinite clay and is mined in Cornwall, England and China.
SANDSTONE Sandstone is a sedimentary rock, an intact mineral of quartz sand. It is
cemented together by the commonest cement, calcium carbonate.
QUARTZITE Quartzite is metamorphic sandstone, an intact mineral or solution of
suspended silica growing on quartz crystal, sand is broken quartz crystal.
LIMESTONE Limestone is a sedimentary mineral cemented by calcium carbonate and is made of once living organisms, the dissolved mineral is calcite. In this
same category of sedimented rock is salt and gypsum. Powered and heated,
"calcined" limestone makes plaster of Paris, mural mortar, we'll get to slaking lime in the mural chapter in Mediums Explained.
MARBLE Marble is metamorphic or recrystallized limestone, being metamorphic is
being made with pressure. GYPSUM Gypsum's are crystals of hydrous calcium sulfate, crystal deposits formed as precipitates from sea water or in limestone, These circulating waters contain sulfuric acid generated by oxidation of sulfur ore minerals. Gypsum crystals are called selenite when there clear, alabaster, when there translucent and fibrous, satin-spar, when there opaque and bendable. Gypsum heated forms sulfur dioxide gas and sulfuric acid. Heated in the
presence of lead, either with fumes or in a natural combinations, will form basic lead carbonate, called lead white. Gypsum, after being heated loses most of its sulfur and becomes plaster of Paris, lime plaster is better to paint on.
HORNFELS Hornfels are a combination of clay and fine quartz sand, forming silt
which makes shale. Pressure forms hard rock hornfels. DIATOMITE Diatomite is diatomaceous earth, a porous chalk like material, a
sedimentary rock that forms on a sea floor or lake bottoms. It's a form of
opaline silica, microscopic plants secrete silica to form hard shells of opal.
Diatomite is a common filler in paint and paper. FLUORITE Fluorite is crystal of calcium fluoride, found in veins with the metallic ores of lead and silver, or with barite, gypsum, calestite and dolomite, or by itself. Fluorite forms a full color wheel, it's the only non-metallic element that
has this capability. There's also a luminescence fluorite which emits a visible
light when crushed, heated or radiated. England mines "Blue John" which was
carved into bowls, cups and vases. The Chinese mined a green variety and carved statues. BARITE Barite is crystal of barium sulfate, called heavy spar, it has no coloring
power by itself. Barite is a non-metallic mineral crystal mined in England, filling the cavities in limestone. As barium it's an extender in lead based, cadmium paints and vat dyes CELESTITE Celestite is strontium sulfate, the ore of strontium. This strontium
element supplies "the rockets red glare". HALITE Halite is sodium chloride, rock salt.
LAKE MINERALS Borax is found in dry lakes as in Tibet, it was called "tin-cal", a
Chinese word. Boron is also found in boric acid and in the mineral sassolite,
mined in Tuscany, Italy. It can be found in a mineral called tincalconite and
ten others. Borax and shellac form the paint called "water shellac". Boron
hardens metals, and makes soaps and medicine. Molten borax will dissolve
insoluble metal oxides and is the flux for soldering, brazing and welding
metals. TRONA Trona is soda ash in its hydrous state as in sodium carbonate. In its
anhydrous state, it's soda ash. 1/5 soda ash and 4/5's sand quartz, make glass,
with enough heat. SODALITE Sodalite is sodium aluminum silicate with sodium chloride, it forms in
massive crystals, like over an acre! SULFUR is abundant in gypsum and as an anhydrite from sea water, it is also found in limestone to some extent. Sulfur was used as an insecticide in 1000 B/C, ancient Greece gathered it in Delphi, from a deep crevice exuding sulfurous gases from Mount Parnassus. Sulfur burns a blue flame and emits sulfur dioxide gas. SULFURIC ACID is sodium carbonate and is used in making glass. It was once called "oil of vitriol", a di-basic acid of sulfur made from sulfur tri-oxide. To "vitriolize", means to treat with sulfuric acid, it gives a glassy appearance to metallic sulfates; white vitriol is made from the lead or zinc ore, blue vitriol is made from copper, green vitriol is made from copperas, a ferrous sulfate, iron. SULFIDE contains the free radical or more electropositive element, it effects changes with other ores. SULFATE, to treat with a salt of sulfuric acid will make a sulfate. Lead sulfate compounds form on lead, you "sulfatize" when you roast galena, the lead ore that contains sulfur. SULFUROUS ACID is dissolved sulfur di-oxide gas in water, to form salts called sulfites. SULFITE is the salt of sulfurous acid which makes the cadmium sulfides from yellow to red. Arsenic sulfides are yellow-green, yellow, orange and red. Copper sulfides are green to blue. Lead sulfides range from white to red. Note: Oil coal-tar colors go from hansa yellow to ultramarine blue moving around the magenta side.
CEMENT CEMENT, like in Portland Cement, is made of lime, from heated gypsum, sand and alumina which is the oxide of aluminum present in clay. That makes cement hydraulic, the clay absorbs water and the cement sets quickly, too quickly for mural work. Heated gypsum burns off sulfur as it becomes lime, any traces left behind would also be bad for a mural's pigments. So don't use gypsum lime for murals, use limestone lime. Soak, or slake the lime from six months to twenty years, the longer the better and add 3 sand to 1 lime to make fresco mortar. Use a fine sand or marble powder for the last coats, three coats are better than one and six coats are better than three. The final painting coat should be less than 1/8th inch thick. CEMENT FONDU is a cement with a high aluminum content, KEENE CEMENT has alum salts added and makes a very hard mass. CASEIN CEMENT, casein added to cement makes it harder and set faster. HIDE GLUE CEMENT, Add less then one percent hide glue into the cement to slow setting from ten minutes to two hours. OXYCHLORIDE CEMENT is also called , PLASTIC MAGNESIA. It's calcined magnesite, it's calcined just short of becoming magnesia (as limestone becomes lime), and made into cement with a strong solution of magnesium chloride. This is CAST STONE. STANDARD COLORS IN CRYSTAL MINERAL COMPOUNDS, CRYSTAL TERMS
ALLOCHROMATIC, is an element coloring term meaning an outside element is included in the compound. ANISOTROPIC, Crystals in which light travels at different velocities in different directions. AMORPHOUS, without crystalline structure, like the opal or glass. CLEAVAGE, a breaking point along a face, variable. CUBIC SYSTEM, four pyramids or tetrahedrites block together and make a cube, this system has the highest symmetry, the triclinic has the lowest. The other systems are Tetragonal, Orthohombic, Trigonal, Hexagonal, and Monoclinic. HABIT, is the shape of a crystal. There are seven systems, and thirty two classes within the systems. PARTING, similar to cleavage but along the twinning axis. PSEUDOMORPHS, A crystal inside a rock may change it's chemical composition and still keep its original shape, common to quartz. FRACTURE, broken.
AGGREGATE, A mineral without an obvious crystal shape, crystals forming masses as gold and silver (dendritic), tree or plant like, or the kidney-shaped hematite (reniform). COMPOUNDS, Two or more elements form compounds. Oxide compounds are combined with oxygen and a metal element, hydroxides, carbonates, silicates, sulfides, sulfates, arsenides, antimonides, Tungstates, and chromates are all compounds. CARBONATES, carbon compounds. GANGUE, other minerals found with the mined ore. GOSSAN, The "iron hat" of gangue minerals with iron and manganese oxides, the soluble minerals sink lower in the earth to form azurite, malachite, and cuprite. Sulfur sinks still lower and forms sulfides like chalcocite. HALIDES, contain halogens, negative elements, fluorine, chlorine, iodine and astatine. NATIVE, Uncombined with other elements, natural. OXIDES, a mineral combined with oxygen as the sole anion. These are usually hard because of close packing. HYDROXIDES, have the complex anion (OH). PSEUDOMORPHS, A crystal inside a rock may change it's chemical composition and still keep its original shape, common to quartz. SILICATES, Silicon combined with oxygen, SiO4. STREAK, The color left behind from an abraded (usually rubbed on frosted glass to test) mineral. SULFIDES or SULPHIDES, Metallic ores formed in the presence of sulfur and the absence of oxygen, C032-. TENOR, The metal content of an ore. ANTIMONY
ANTIMONY native is Naples yellow, a very early pigment replenished in the Vesuvius eruption of 79 A/D. Antimony oxide was made artificially since the early eleventh century. Antimony sulfides are found in the mineral galance stibnite, in Italy and Human, China. ARSENIC
ARSENIC, Arsenopyrite is arsenic's main ore. Arsenic disulfides are red realgar and yellow orpiment, both were used in the early Egyptian days in paint. COPPER
COPPER ORE native, is brittle unless it's heated (annealed) as the ancient Anatolias did in 6000 B/C. The ancient Eskimos ground fish hooks out of this native copper. Copper pitch ore was used all through the Neolithic Period. CUPRITE is a cubic transparent ruby-red crystal, formed as a secondary mineral from exposed copper ore. TENORITE is black copper oxide. CHALCOCITE is another secondary mineral of copper, found in metallic sulfides, precipitating in still waters as a soluble sediment. CHALCANTHITE is retrieved from the chalcocite sediment, this is the basis of the ancient blue vitriol, and it made blue and green frit. Notice, it's the complementary color of cuprite. I'll point this out in color wheel oppositions. ANTLERITE is copper's chief ore mineral, it's a copper sulfate. CHALCOPYRITE is the primary ore formed in quartz. CHRYSOCOLLA has a hardness of 2.5, it's a light cyan colored ore that the Egyptians wore as jewelry, they matched this color with copper and tin glass frit. Small scarabs were found glazed and considered as good luck pieces, it was a sample of the color pigment they sold on the ancient world market. Or crushed it was the pigment itself. MALACHITE is a secondary mineral ore of copper carbonate, a native green pigment when crushed. AZURITE is another hydrated salt of copper carbonate, this one's a deep cyan color which looks blue, it was also used as a pigment all throughout the ancient years, it was transparent to opaque. Soak azurite long enough and it will turn green, ammonia turns copper blue. ACETATE is a salt by acetic acid or vinegar. A hydrated copper acetate was said to be the first artificial color, I made some and it was cyan on the green side. It was made by the Romans in 1000 B/C and was called vertigris. They would have had to suspend Egypt's chalcocite sediment in vinegar to precipitate a salt. Egypt had murals of wine harvests 2500 years earlier. I suspect Egypt made it first, before Rome, since they did so much with copper. PHTHALOCYANINE is copper with one of it's atoms removed to make a non-metallic pigment. This pigment doesn't react to sulfur as metallic copper does, it is transparent and covers the colors from yellow-green to cyan. This is perhaps the most important color ever discovered. IRON
GOETHITE is iron hydroxide crystals found yellow to brown in earthy masses, it's an ore of iron that contains the natural yellow ferric oxide pigments. IRON OXIDE ranges from white calcite, yellow, yellow ocher, sienna, red oxide, brown and green umber to black. Adding heat (calcine), brings out the red side and adds a transparent quality if silicates are involved, as in sienna. Magnesium is the purple side of caput mortuum, present in iron. HEMATITE is iron ore, heat it to 1830 degrees and get iron. Hematite naturally crushed and oxidized leaves a red streak powder that was the stone-age red and Egyptian rouge. PYRITE is iron sulfide and sulfuric acid, dissolved in water to leave behind limonite. Fifty four percent of pyrite is sulfur, decomposed pyrite is hydrated iron oxide. LIMONITE is a secondary ore of iron that forms yellow to brown oxides. This is the second bit of proof that yellow and brown are the same color (Tint to shade) in my color wheel, CENTERING COLORS. SIDERITE is iron carbonate, going from yellow to brown. CALCITE is a native iron pigment mineral that is white. Iron will make all colors. CALADONITE is a green earth iron silicate mineral. LEAD
GALENITE or GALENA. Galena is lead sulfide mineral, native, and it contains sulfur. Roasting the galena ore produces a basic lead carbonate, white lead sulfate. Also formed is a water soluble salt compound of lead arsenate. CERUSSITE is a native lead carbonate mineral, white lead. VANADINITE washes to lead vanadate, a red-orange crystal. WULFENITE is a transparent orange crystal. LEAD ACETATE is a colorless crystal formed by acetic acid touching lead, it is water soluble and called sugar of lead. Lead acetate can be used as a siccative. LEAD IS CORRODED with fumes of acetate and carbonic acid in steam. It forms a white lead sulfide or oxidized white lead and contains the free-radical that effects so many pigments. This is artificial basic lead carbonate, our white pigment. Hydrogen peroxide will stabilize and remove the free-radical, turning lead sulfide to lead sulfate, a stable white lead. LEAD CARBONATE is formed by mixing in solution, lye and carbonic acid with lead acetate. It's the whitest lead but not as opaque as white lead oxide. MERCURY
CINNABAR is the ore of mercury, mercuric sulfide, the red crushed ore is the color vermilion. Cinnabar is found in crystal and masses in South Central Spain and in China. China did more carving in it than painting with it. The rest of the world found it a very useful pigment. The Carthaginians and the Romans both worked the Spanish mines for cinnabar. TIN
TIN ORE is found in cassiterite, a tin dioxide, the principle ore of tin, along with wood tin. The symbol for tin is Sn, for stannum. Tin ore is found in rock forming minerals like feldspar, quartz and mica. First, tin was exposed on cliff faces, where the rocks eroded exposing the veins. Eventually it entered streams and rivers to be stream mined. Tin was mined in Eastern Greece, ancient Anatolia, Upper Syria, the Tigris Euphrates Valley, Afghanistan, the Mekong River and England. Egypt's paints were tin based. TIN OXIDE is black and fires white, mix tin oxide and cobalt oxide with heat and you get cerulean blue. You can get a similar blue by mixing and firing tin and copper chalcanthite with quartz sand like the Egyptians did, this made their highly prized frit colors, which they traded through the Phoenician's, ancient-world-wide. Smelt tin ore and copper ore and you'll get bronze. Bronze is the metal the Egyptians made their saws out of to cut the limestone for their great pyramids. ZINC
ZINC: The zinc primary sulfide mineral is water soluble, and is often found with galena. ZINC SILIATE: Zinc silicate comes in a variety of colors, transparent yellow and green, opaque white,
ZINC OXIDE: Zinc oxide is called zincite and is colored white, yellow, orange and red. B/C MINING
40,000 B/C- Tribes mined flint in Egypt and France. 6,000 B/C- Neolithic communities like Anatolia hammered copper, Copper works were found in Catal Huyuk, a culture in Turkey. 6,000 B/C- Eskimo's N W of Hudson Bay had copper fish hooks made from glacial copper. 5,000 B/C- Pre-dynastic Egypt mined gold, silver, chalcedony, (a milk colored quartz), chrysoprase, a nickel stained apple-green chalcedony, green feldspar, (light-green amazonite), green fluorite, malachite, (crushed malachite was their green eye shadow paint). Hematite was red rouge. Lapis lazuli was jewelry. 4,700 B/C- Egypt's IV Dynasty smelted bronze. 4,000 B/C- Egypt's casting gold, copper and bronze, bronze saws cut the blocks of lime stone for the pyramids of Giza. They burnt gypsum and limestone for plaster and cement to cover walls, make columns, and as supports for murals. An Iron and nickel alloy knife was made and found, probably from a meteorite. 3,400 B/C- Afghanistan and Mesopotamia were mining lapis lazuli. 3,000 B/C- Galena was found in every country from Morocco to Greece, from Russia to China. Neolithic miners dug for flint in England, a small figurine of a pregnant woman was found at the back of a limestone cave. I wish I had saved the photo. Old Kingdom Egypt was painting murals of miners, smelters, farmers and "the good life". 2,700 B/C- Gold found in the Royal Caves of Ur, sprinkled on the dead. 2,000 B/C- The Nubia people mined 1,000 tons of gold for Egypt. The Phoenician's smelted galena and silver. Bronze was all over the Mediterranean and China. 1,500 B/C The Hittites of Anatolia smelted iron, Zinc was smelted from lead. 1,350 B/C King Tutankhamen, of the XVIII Dynasty had smelted tools of iron, a Life-like gold casting of him was in his tomb. 1,100 B/C The Phoenician city of Gadez, became a tin market. 1,000 B/C Greece was smelting bronze also. 1,350 B/C King Tutankhamen, of the XVIII Dynasty had smelted tools of iron, a Life-like gold casting of him was in his tomb. 1,100 B/C The Phoenician city of Gadez, became a tin market. 600 B/C Clay plate painting of "miners" at Corinth, Greece.
B/C PIGMENT PALETTE Here's the mineral palette back when Sandraca (sandracca) was King and Zeus had a son named Castor. Castor is also the brightest star in Gemini. Castor oil was added to sandaraca (sandracca) about 2000 B/C, to soften, extend and make it pliable. Sandaraca was a major medium into the A/D's, when mastic, wax, egg, and oil started replacing it. WHITE
ANTIMONY GALANCE, stibnite ore was roasted or found native. GYPSUM, native calcium sulfate, calcined to 250 degrees. IRON, native ore calcite, oxide. LEAD, Lead white, "ceruse", basic sulfate of lead, native or burnt galena ore. LEAD, "White vitriol", made with sulfuric acid fumes. LEAD, Lead white carbonate oxide, made by acetic acid fumes and carbonic acid fumes on lead in a closed container. MAGNESIUM, carbonate found native, the whitest ore pigment. MICA. Silicate laminated natural, native. Japanese pigment. SHELL, powered. TIN. Calcined tin oxide. ZINC oxide found native. ZINC "White Vitriol", made with sulfuric acid fumes. BLACK
BONE and HORN. Roman "atramentum", charred deep black. CARBON. Oil soot, lamp black. IRON. Iron oxide, native. MANGANESE. Black manganese dioxide native, ancient "pyrolusite". ROCK, Slate gray, crushed. SULFUR. Ultramarine ash, the first washing contains some matrix rock, this leaves a cool opaque gray. YELLOW
ARSENIC. Orpiment, arsenic sulfide, native, Egypt early, 3000 B/C. ANTIMONY. Naples yellow, native, 500 B/C, replenished 79 A/D in the Vesuvius eruption. IRON. Yellow ocher natural, "minette", "sil", "chamois", heated to gold ocher, flesh ocher and red. IRON. Raw sienna, heated to burnt sienna and translucent vermilion. IRON. Amberg yellow, a very bright fresco yellow ocher no longer available, native. LEAD. Lead oxide white heated to a cool yellow, "Massicot", "King's Yellow", "Cassel Yellow", lead also heated to orange, red and brown. ORGANIC-ANIMAL. Indian Yellow is magnesium euxanthate, an early lake used in sandaraca, mastic, cara cola, water and oil pigment until 1899. Made from cow's urine, India. Two colors, yellow to brown and orange to yellow, transparent. 1800, England, COLOR, Indian Yellow, the best and very permanent transparent yellow was brought to England from India, where it had been used as
a pigment for as long as India had cows. The raw product is called Monghyr, magnesium euxanthate natural organic, after a city in Bangal. England made this
in oil and kept it's ingredients a secret for eighty years. It was in brown to yellow and orange to yellow, two dual-toned colors.
ORGANIC-PLANT. Tree sap, "gamboge" transparent, alcohol base, Thailand. ORGANIC-PLANT. "Turmeric" root, "curcuma" root, transparent yellow to brown, India, Asia. ORGANIC-PLANT. "Saffron", flower power, bright yellow, India. TIN. calcined from white to pale yellow to pale magenta. ORANGE
ARSENIC. Realgar, arsenic di-sulphide, native, Egypt 3000 B/C. "Risalgallo", Roman, red-orange clear crystal. IRON. In clay, burnt sienna, high in silicic acid until calcined from raw sienna, than it's high in silica and is transparent. TIN, calcined from pale yellow to pale magenta. ZINC. Zincite, native zinc oxide, ore of zinc, a brittle mineral ranging in color from yellow, orange to deep-red, opaque. RED
COPPER. Cupric crystals native, transparent red. HEMATITE IRON, ore, native red streaks of iron oxide, where abrasion has ground off hematite ore in place. IRON, in clay, "cinabrese", Cennini described a native light vermilion red that is now exhausted. IRON, in clay, "Armenian bole", red ocher. IRON, in clay, "sinopia", native red oxide, Roman 100 B/C IRON, in clay, "sinopis", a very light red ocher from Asia Minor, exhausted. LEAD. "Minium", red lead oxide is made by heating white lead in the presence of air, turns dark in fresco as all leads do, this was a mastic, oil and wax pigment that is unaffected by alkalis. Phoenician, 1000 B/C, Greek, Roman, 500 B/C. MERCURY. "Cinnabar" native, is the ore of mercury. "Vermilion" natural was one of the two most prized and expensive pigments of the ancients, not counting gold. Mercuric Sulfide. ORGANIC-PLANT SAP. Brazilwood lake, blood red transparent. "Dragon's Blood", ruby red lac, Singapore, as ancient as karmes. ORGANIC-PLANT FLOWER. Safflower red, "Carthame". ZINC. Zincite red, native red oxide zinc ore. MAGENTA
COBALT. native is a pale violet-magenta color. Cobaltite is a native mineral ore, cobalt arsenic sulfide, it's an opaque cool magenta color. IRON. in clay, "Pozzuoli Red". A rosy opaque magenta, Roman. ORGANIC-ANIMAL. "Carmine", cochineal insect, a transparent dyestuff precipitated on clay, also roasted darker. "Karmes" another insect, similar color, 2000 B/C or earlier. "Nacarat carmine" is the highest quality color. ORGANIC-PLANT ROOT. "Madder lake", boiled Rubia Tinctoriun root on clay or transparent water based in wax soap, Egypt, Greece. Light rose to dark magenta, not for fresco. TIN. Highly heated tin oxide makes a pale magenta. BROWN
IRON. Burnt green earth, ferrous hydroxide and silicic acid, transparent like sienna. IRON. "Caput mortuum" is red ocher or oxide calcined, or native. IRON MANGANESE. Raw umber, "umbra", "terre d'ombre". Manganese dioxide and iron hydroxide. Calcined raw umber makes burnt umber. PURPLE
MANGANESE. Manganese violet oxide, native. ORGANIC-ANIMAL. "Tyrian" is Greek, "Ostrum" is Roman, "Byzantium", are all names for purple, from the Murex shellfish family. The color ranged from pink to blue transparent. According to Pliny it was the celebrated "Imperial Purple" of the Romans. BLUE
COBALT. Black oxide of cobalt fires cobalt blue. China 2500 B/C on pottery, 500 B/C Roman, maybe earlier, they made a smalt after the Egyptian frit of copper. COBALT-TIN. Cobaltous stannate, cobalt and tin oxide in potash glass, light cobalt blue colored smalt, Egyptian or Greek or Roman. COPPER. "Azurite" natural, blue-cyan, Egypt 3000 B/C, "chessylite", hydrous copper carbonate. COPPER. Copper hydroxide plus copper carbonate, "blue verditer", "mountain blue", "Bremen blue". COPPER. chalcanthite, "Blue vitriol", copper salts and sulfuric acid fumes make a copper sulfate. IRON. Pompiian blue lake, a ferris-cyan, Roman 100 B/C. SULFUR. Lapis lazuli native, 3000 B/C, sodium sulfosilicate ore. CYAN
COPPER. Chrysocolla, a native copper silicate, first a pigment in Egypt, than a jewel, crushed glass-frit than became a pigment of the same color. COPPER. "Egyptian blue" 3000 B/C, copper silicate transparent, "Pozzuoli blue". COPPER. Frit, copper salts fused in potassium silica glass, Egypt 3000 B/C. COPPER. Verdigris, hydrated copper acetate crystals, water or resin soluble. This color is usually listed under green, (vert) meaning green. Since this was the first artificial color made by the Romans, I made some. Using materials I knew they had I put copper in ammonia, this turned the ammonia blue, a few drops of acetate acid (vinegar) and the color changed, it precipitated a light cyan-green salt. Mixing the salt with damar, I painted with it, and, well it does go a little farther, I mixed the salts in sulfuric acid and it turned clear. I was stirring the mix with my steel palette knife and it put a copper plating on it! I wonder if the Romans ever rust proofed any of their iron. I remember reading once, that at an Egyptian excavation they found some clay pots with holes in them for wires, the archaeologists thought they were using them as storage batteries for plating, I believe it. MANGANESE. Manganese also makes a cyan hue, I don't know when it was first used, but it was sold as a liquid in a blatter. ORGANIC-PLANT. Indigo, India. Woad, England. Both transparent cyan dyes, Indigo was the better.
GREEN
COPPER. Malachite, ore of copper, copper carbonate. Crushed into pigment. IRON-COPPERAS. ferrous sulfate, "green vitriol", transparent. IRON. Ferrous hydroxide plus silicic acid, native, "Veronese green earth", "tirolean", "bohemian", translucent.
YELLOW, Imperial yellow is from the flowers of the "sophora japonica", it contains flavonal quercetin, similar to the famous Indian Yellow, both had staying power and were a golden-yellow color when used full strength. Yellow wood sap from the sumac tree, "rhus cotinus" works, flavone also occurs in vines of weld, from Northern India. Four other sources of yellow are; safflower and saffron, the root of the "curcuma tinclora" and the husks of pomegranate with carbonate of zinc. ORANGE, henna "lawsona alba". RED, Cochineal, ground female "coccus cacti" insect, originally from Central America, imported to Morocco. Soluble in ammonia. The coloring matter is carminic acid, an anthraquinone derivative. MAGENTA, Karmes Scarlet is the oldest Magenta color, made from an insect found on the oak tree, it secrets an alcohol based lac and is found all over Europe. MAGENTA, Madder root from the "rubia tinctoria" from red to magenta to brown. Found from Anatolia to Persia. India and China use the "rubia cordifolia", which is a cooler magenta color. India exported madder, indigo, weld and Indian Yellow. The three primaries plus an ultramarine blue color. MAGENTA, Brazilwood, named the country, its color is clear in wood and boiling it makes a magenta dye, to dye red, you add a tin mordant, Brazilwood dye comes from the local "caesalpinia" tree, Logwood, from the "haematoxylon" tree makes hematin, boiled, it turns violet to blue-black. CYAN, Indigo, Grown in India, the "Indiagofera tinctoria" thrives in the tropical climate, the active ingredient is found in the leaves, an indol derivative is fermented from a sugar, this precipitation is insoluble in water. Alkalis dissolve it and form the sodium salt indigo white, which oxidizes into many shades of blue. Aniline blue has the same chemical composition and replaced it in 1870. This cyan/blue was the most important color in Chinese rugs. MATCHING 36 TUBE OIL PIGMENTS TO RGB WITH COLOR EXAMPLES Here are some permanent chemical pigments used in oil and acrylic paints today. Indian Yellow is a set of two colors, transparent yellow-orange to yellow and transparent raw sienna hue to yellow. These two transparent duel-toned colors mix all greens and reds. Golden Artist Colors, New.. On 6-10-06 I received a welcomed new color from Golden. Indian Yellow Hue, made with Arylide Yellow PY7, Nickel Complex Azo PY150 and Quinacridone PR206. This is good Golden Indian yellow Hue. Also a Brown/side Indian Yellow called Nickel Complex Azo, plus an Indian yellow Hue green/side Nickel Azo Yellow, PY150, PG36, PY3. In the printed copy describing this new Historical Fluid Acrylic Color I was happy to see my information which was first put on the internet in 1996 regarding original Indian yellow. My print research turned up the cow urine history of this very important color and this information started spreading in 2002 and Indian yellow hue started appearing. The internet loves information and Golden used it describing their new Indian Yellow Hue on their Historical Fluid Color Chart. Today is 2009 and the many ways and variances of Indian yellow are being thrown at the wall to see what sticks.
PY153 dioxine nickel complex = Indian Yellow Golden. BEST PY150 azo nickel complex = Indian Yellow Brown/side. BEST PY3 stable di-arylide = Yellow Lemon on barium sulfate, Gamboge, Indian Yellow PY83 stable di-arylide = Yellow Deep, Madder Lake, Alizarin Crimson, Italian Brown Pink Lake. PY83 stable di-arylide HR = Indian Yellow PY153 dioxine nickel complex + PO69 isiondolin = Indian Yellow Golden. PR 260 isoindolin = Indian Yellow Golden, also in Vermilion to Red Scarlet, transparent but not dual-toned PY129 methin copper complex = Golden Green, Indian Yellow Green with PY153 PR101 synthetic iron oxides = Translucent Yellow to Brown
PY153 dioxine nickel complex + PR260 or PO69 isoindoline = Indian Yellow Golden.
PY153 dioxine nickel complex = Indian Yellow Golden. BEST
needed for their opaqueness and brilliance. PY35 cadmium Zinc sulfide = Cadmium Yellow Lemon PY37 cadmium sulfide = Cadmium Yellow Light, Medium PO20 cadmium sulfo-selenide = Cadmium Orange PR108 cadmium seleno sulfide = Cadmium Red Light, Medium PR101 synthetic red iron oxide = Red Oxide PY42 synthetic yellow iron oxide = Yellow Oxide PBr7 natural iron oxide, raw and calcined = Siena and Umber PB29 silica, aluminium, sulphur complex = Ultramarine Blue PB28 oxides of cobalt and aluminum = Cobalt Blue PG17 anhydrous chromium senquioxide = Chromium oxide Green 36RCW#1 is the first color arc, RCW#1.0 is the full color in the first color arc, RCW#1.0.1 to RCW#1.0.9 are the warm and cool of the full color, RCW#1.01 to RCW#1.09 are tints of the first color, RCW#1.1 to RCW#1.10 are the darker scaled colors of the first arc. The RCW Pigment Color Number for Cadmium Yellow Lemon Opaque is:
Brown is the darkest intensity of yellow-greens, yellows and reds. Mix this low intensity brown with its opposite color Ultramarine blue to Cyan for a neutral dark. 36RCW#1.01 to RCW#36.01 are the outer rings, lighter pigment or tinted hues of this color section.
#1.01 Tint of the 1st color arc, yellow
To reach a dark neutral use the darkest form of yellow, orange or red (all of which are burnt umber) to mix with its opposite color.
Darken a yellow object first with graduated color oxides like yellow ocher light, medium or dark.
Cyan first darkens to blue, like the sky.
The rest of this color wheels colors will darken by adding the opposite color until it reaches neutral dark.
Therefore, anyone working with pigments can reliably find truly opposite colors, which will always mix to a neutral dark without using black pigment.
ACID- Boric acid is a very mild acid, as is acetic acid. Carbonates and sulfides of metals are sensitive to mineral acids. Acid vapors and weak acids bleach lapis lazuli and syn. ultramarine blue, a colloidal sulfur. Acid liberates hydrogen ions, an electrically unbalanced or charged atom, Hydrochloric acid is violently caustic on metals. Sulfuric acid is weaker then hydrochloric acid. ALKALI- Alkali or base, is caustic and reactive, sodium hydroxide is caustic soda or lye, both lye and ammonia are strong alkalis. Alkali yields to hydroxyl (OH) ions, the opposite of acid. Sal soda is a sodium carbonate or washing soda or soda ash, it's weaker but it will still dissolve wool and react with oils fats and wax. Sodium bicarbonate or sodium acid carbonate is baking soda, the dry mix of alkali and acid neutralize each other when their hydrated, and carbon dioxide is set free. Borax is sodium tetraborate, a mild alkaline salt that's used as a flux with glass. ALUM- A double sulfate of aluminum and potassium crystal, a 5% solution will harden hide glue, gelatin and all proteins. It makes cement hard, and is a mortar for dyeing textiles. It's an astringent, alkali. ANHYDROUS- To lose all water, including crystallizing waters. ANHYDRITE- A mineral, calcium sulfate. ANILINE- An oily liquid first obtained from indigo, used as a basis for aniline dyes, later coal-tar was the base. ATOM- The smallest unit constituent of an molecule containing protons, neutrons and electrons, the number and arrangement of which determine the element. AZO- Unsulfonated dyestuffs, diazonium salt with a phenol, Hansa Yellow. CADMIUM- A metallic element that looks like tin. Chromite cadmium from lemon yellow through, yellow light, yellow medium, yellow deep, yellow extra deep, yellow orange, orange, red scarlet, red light, red medium, red deep to red purple. CALCINATE- Calcination means to heat, burn or cook, Calcinating a salt of iron makes an iron oxide, slow oxidation is rust. CROMA- Color saturation of a hue. CHROMITE- The principle ore of chromium containing iron and magnesium. CHROMITE- A salt of chromous acid, a coloring agent. Chromite white lead, from yellow through orange to red lead. CHROMATE- A salt of chromic acid with a radical atom that will easily leave it's host molecule. To make a chromate, you change a chromogen from colorless to colored. A chromate of lead is red lead. Chrome red, or red lead is basic chromite of lead treated with chromium salts of chromic acid. CHROMOGEN- A substance that colors when oxidized, forming colored compounds, like Azo. CHROMIUM- Element, metallic, occurring in compounds to make pigments, a hardening element as with chromium steel. Chromium compounds are chromic acid and it's salts, called chromates. First the metallic and acid name, followed by (ATE), sodium sulfate. This is the salt of sulfuric acid and a sodium compound. The salt of acid with fewer atoms to the molecule, ends in (ITE), sodium sulfite, the sodium salt of sulfurous acid. (IC) and (OUS) designate numerical variations in an acid or oxide, (OUS) being smaller and more unstable, these that don't contain their maximum complement of atoms are called unstable. (THIO) means sulfur-bearing, (AZO) means nitrogen-bearing, CHROME- The adverb for chromium, chrome yellow is composed of chromates of lead, barium, or zinc, Chrome green is made from chromic oxide, chrome red is a basic chromate of lead. CHROME- A word element meaning color. CHROMOPHORE- Any chemical group which produces color in a compound as the azo group -N=N-, the structural layout of atoms which is found in many colored compounds. COMPLIMENTARY COLORS- A color and it's opposite on the "real color wheel" will combine to a neutral dark on the pigment color wheel and white on the light color wheel. To find the complimentary by eye use your color reversing image retention. Look at the color swatch in a bright light for 5 seconds, than switch to a blank white paper. The reversed color will appear. COMPOUNDS- Composed of two or more elements or ingredients. Binary compounds consist of two elements, first named is the metallic or electrically positive, followed by the negative, which ends in (IDE), sodium sulfide is a binary compound of sodium and sulfur, zinc oxide is zinc and oxygen. CRYSTALLIZATION- with water, as the blue transparent crystals of copper sulfate contain seven molecules of water to each molecule of salt. Without the water it's an anhydrous white powder. CYANOGEN- A gas with a univalent radical, added to iron gives ferricyanide salt or Prussian blue. DICHROMIC- A color exhibiting two color phases, usually a transparent color that looks different when white is added as opposed to adding water. DICHROMIC- Chemical, of a compound containing two atoms of chromium. EFFERVESCENCE- to give off bubbles of gas, as when mixing heat, ammonia and beeswax while making wax soap. EFFLORESCENCE- means losing water to air. ESTER- Ester or salt, containing glycerin is a glyceride. Oil is a glyceride of fatty acid from plants, glycerides in drying oils are unsaturated and combine with oxygen forming insoluble linoxyn. ESTER- A product formed by the reaction of an acid with alcohol, silicon esters are ethyl silicate (alcohol and silica), volatile. Adding water has a chemical reaction called hydrolysis, producing hydrated silica, a stone preservative that is inorganic and imperishable. This is a complete painting medium, great for outdoor murals. It has an alcohol cleanup until it's dry. Ethyl Silicate is made by Union Carbide Company. FATTY OILS- Drying, vegetable oils, linseed, poppy and walnut oils dry by absorbing oxygen, siccatives speed up the drying process. All oils turn yellow. FORMALDEHYDE- the best hardener for egg, casein, hide glue or any protein. A 40% solution of formaldehyde is called formalin, a 10% solution of formalin is sprayed on the dried protein to harden it. Formalin is a fungicide and prevents mold. HYDRATION- To lose water as hydrated oxides do when exposed to heat. HYDROUS- Containing water as in hydrates or hydroxides. HYGROSCOPIC- means to absorb water from the air, like calcium chloride, alcohol, clay and lye. INERT- Inert has no action to effect changes to itself. INORGANIC- Minerals and ores are inorganic and inert. MASS TONE- The color perceived by laying down a thick coat of pigment. ORGANIC- Plants and animals are organic. An organic salt is called an ester. MOLECULES- The smallest physical unit of an element or compound consisting of one or more like atoms in the first case, and two or more different atoms in the second case. Molecules of red iron oxide are; Fe2, O3, Fe = iron, O = Oxygen, 2 equals two iron atoms, 3 equals three oxygen atoms. Synthetic resins rearrange the molecules. RADICALS- Two or more elements hook valences together and act as a single element in a reaction, as (HO) is hydroxyl. SALTS- Salts are neither acid or base, but they could have those reactions. Salts are produced by action on metal or another salt, or by the neutralizing reaction of an acid or base. Sodium chloride is table salt, it's soluble, inert and neutral, some salts are active. Barium salt is insoluble. Some pigment salts are made by mixing two solution salts together, forming two new products (called double decomposition), one will remain soluble, the other precipitates as a powder, as some pigments do. Salts of metals categorize themselves in color, nickel is green, copper is blue, cobalt is rose and anhydrous blue, chromium is yellow to dark red and iron is from yellow and red to brown. This note is taken in my color theory of rim and centering colors in elements. Normal salt of acid is named for the metal then the acid, ending in (ATE). Sodium sulfate Na2 SO4 is sodium salt of sulfuric acid, combined. The salt of acid with fewer atoms to the molecule ends with (ITE), sodium sulfite NA2 SO3. Unstable or unsaturated acids and salts have open hooks and end in (OUS). Ferrous (iron) oxides are exceptions, these are called "loose linkages". SICCATIVE- Salts of metal oxides soluble in oil, they speed the absorption of oxygen by oils and dry them faster, 2% maximum is recommended for pigments. SOAP- Made by treating a fat with an alkali, any metallic salt of an acid contained in fat. SODIUM- A soft metallic element that oxidizes rapidly in moist air, occurring only in combined states. PHENOL- Carbolic acid, a hydroxyl derivative of benzene, used in organic synthesis. Phenolate, a salt of phenol. POTASH- Potassium carbonate, obtained from wood ashes. Caustic potash (pot-ashes). POTASSIUM- Similar to sodium element, used in hard glasses. POLYMERIZATION- means internal changes. POTASSIUM BICHROMATE- hardens proteins, it's a fungicide. REACTION- An irreversible chemical change, as when oil dries. UNDER TONE- The thin wash color of a pigment, as opposed to the MASS TONE or TOP TONE. VAT PIGMENTS- include, perylenes, indanthrones, phthalocyanines, and quinacridones. They have no body. VALENCE- Valence is a hook on an atom that links two atoms together, the amount of hooks is the valence number. Oxygen has two, carbon has four, the benzene radical has six.
1. Antimony = Naples Yellow 2. Cadmium = Yellow, Orange, Red 3. Chrome Green = Green Chrome + Alumina = Transparent Corumdum Red Chrome + Cobalt = Blue/Green Chrome + Tin = Pink (light Magenta) Chrome + Tin + Silica = Red Chrome + Tin + Calcium = Red, Magenta, Violet Chrome = Tin +Tin + Cobalt = Ultramarine Blue, Purple, Violet 4. Chromium = Green Opaque Chromium + Iron + Manganese = Black Chromium Trivalent = Green Chromium Hexavalent = Yellow 5. Cobalt = Azure Blue Cobalt = Uranium = Green Cobalt + Zinc = Ultramarine Blue Cobalt + Chromium + Manganese = Black 6. Copper = Green, Turquoise, Red, Ruby Red Violet Copper Oxide = Green Copper Oxide + Zinc = Brilliant Green 7. Ferric Oxide Lead Silicate = Yellow Iron = Green, Yellow, Orange, Red, Brown, Black, Cyan, Ultramarine Blue Iron Oxide = Opaque Red 8. Gold = Magenta 9. Lead = Yellow Lead + Chromate = Red Litharge = Red Minium (Roman) 10. Divalent Manganese = Yellow to Brown Manganese = Brown, Red, Magenta, Violet, Purple 11. Magnetite = Black 12. Molybdenum = Smokey Gray to Blue 13. Nickle = Gray, Blue, Purple, Green , Yellow, Brown Nickle Oxide = Slate Blue Gray 14. Potassium Oxide = Yellow Green 15. Platinum = Silver 16. Silver = Dull Silver Silver Chloride = Yellow Side Silver 17. Selenium + Cadmium + Sulphur = Red Selenium + Cadmium = Orange Selenium + Sulphur = Yellow 18. Salt fires Glossie 19. Tin = White Tin + Chrome = Crimson Tin + Vanadium = Yellow 20. Titanium = Opaques 21. Uranium = Red, Black 22. Vanadium = Emerald Green, Yellow Green, Yellow, Orange, Red, Brown 23. Zirconia = Pink, Magenta Zirconium + Vanadium = Cyan, Turquoise 24. Clay = Glossie Red Oxide (Terra Sigillata, Roman) 25. Clay = Black (Terra Nigra, Roman) ORE'S COLOR REACTIONS TO EACH OTHER ANTIMONY
ANTIMONY isNaples yellow, Antimony oxide white, is Timonox, a British trademark, 1920. It reacts less than lead white with sulfur, has none of the drawbacks of zinc and would give titanium the best run for the money, plus, we would still have Naples yellow, a very dense yellow to light skin color the portraitist used as a base instead of white. If we had a union we would bring antimony back along with Indian yellow, brown side and orange side. ARSENIC
ARSENIC native yellow is orpiment, a sulphide of arsenic. Native red arsenic is realgar, an arsenic di-sulphide. These are ancient colors not available today, the best crystals looked clear and transparent to me. Arsenic tri-sulfide is made today if you can find it. COBALT
COBALT turns black with sulfur. COBALT Oxide is black, calcinated it becomes cobalt blue, fired in potash glass it becomes smalt, a popular early Roman color. COBALT blue today is a mixture of cobalt oxide, aluminum oxide and titanium white, not the same color and not really necessary to the palette. When a color can be mixed using two pigments, you don't need it, but it could be handy as in the brighter Ultramarine Blue. COBALT violet is a very important cool magenta, (cobaltous phosphate) by Bocour, New York, is the best I've ever seen, there is also a German cobalt violet dark, (cobaltous oxide arsenate) if they still make it. COBALT salts with potassium nitrite make the color aureolin yellow. COPPER AS PIGMENT
COPPER turns lead, zinc lithopone (zinc sulfide on barite) and mercury, black. COPPER is turned blue by alkalis like ammonia, the color is precipitated from the ammonia with acetic acid, vinegar. This makes the ancient Roman color, verdigris. COPPER turns black with sulfur. COPPER ARSENATE is emerald green, the most poisonous of all colors. COPPER CARBONATE BASIC is malachite green native and azurite blue native. Native colors are usually unaffected by anything at all, they are inert. COPPER non-metallic phthalocyanine cyan to green should replace all other copper colors, they are inert and safe to use. IRON
IRON RESISTS LYES, SULFUR AND ACIDS AND MAKES THE MOST COMPLETE COLOR WHEEL OF ALL THE ELEMENTS, BY ITSELF. IRON is ferric oxide, iron and the gas cyanogen make ferrocyanide, a salt of ammonium ferrocyanide, makes Prussian blue. IRON hydroxide's are the yellow to brown ochers, based in clay. IRON oxides are the calcined yellow to brown ochers, turned into red ochers or red earths, even higher heat would bring up the violets, until finally, caput mortuum, a purple brown. Raw sienna is an iron hydroxide, burnt sienna has been calcined, higher heat would make a vermilion transparent because of the high silicic acid content in the clay.
LEAD SOAPS TURN YELLOW IN OIL, POPPY OIL IS THE LEAST YELLOWING BUT THE SLOWEST DRYING OF THE DRYING OILS, WALNUT OIL IS NEXT, THAN LINSEED OIL. LEAD sponificates or turns clear in oil, apply lead thickly in the last coats or with age you will see through it to a lower color. Today's lead white is much thinner and slower drying than in the old days. LEAD has a free radical atom that will leave the lead and turn some colors black, this is white lead sulfide. Hydrogen peroxide will stabilize and remove the free radical, turning white lead sulfide into white lead sulfate, a stable white. Roasting lead ore, glance, will also produce basic lead sulfate, like the ancients did. LEAD will turn black with hydrogen sulfide, and permanently yellow with heat. Like the ancient massicot yellow, and King's yellow. It will also heat to orange and red. The red lead is the fastest drier. LEAD can not be used in fresco, the lye turns white lead brown, permanently. Nor can it be used in water colors or pastel, the sulfur in the air will turn it dark. LEAD is very poisonous, Titanium white is the all around better color, with zinc white a close second, but it is too brittle. Combine lead and zinc and you get the best of both. LEAD turns these colors (metals) black; tin, copper, cadmium's (they are sulfur colors), ocher's and earth's if poorly washed will also contain iron sulfates, Nickel yellow and nickel Naples yellow, they don't seem to make antimony Naples yellow any more, Arsenic will also turn black. MANGANESE
MANGANESE native black oxide, manganese carbonate white, manganese green, blue and violet are all good dryers and good colors. MERCURY
MERCURY and sulfur make vermilion, the natural pigment cinnabar is no longer available, today's synthetic vermilion's doesn't contain either element, so there safe to use in any combination. MERCURY, basic sulfate of mercury is bright yellow and turns black with sulfur and copper. SULFUR
SULFUR will turn all lead colors black, also tin, copper, cobalt, cadmium's, manganese, arsenic and antimony.
TIN oxides turn lead black, it works with all other metals. TIN oxides are black, by calcination they become white, tin oxide (stannous chloride) plus cobalt oxide (cobalt sulfate) fired together become cerulean blue, tin and copper fired together become an even cleaner cyan tint, like the Egyptian frit. TIN chromate (stannic) is yellow mineral lake.
ZINC oxide white covers less well then lead white, but does not yellow as lead does. It works well in water color. Hydrogen sulfide reacts to make a different white, zinc sulfide from zinc oxide, there both good, Zinc can be made yellow, orange or red. ZINC does not work with fresco or tempera emulsions, lyes and acetic acid effect it. ZINC white and sulfuric acid make white vitriol transparent. ZINC fades Prussian blue, cadmium yellow, cobalt yellow and coal-tar
pigments when used in water colors and gouache.
DEFINITIONS, TERMS, GLOSSARY
ATOM, smallest particle of an element with all it's properties. ATOMS, are measured in Nanometers, (1nm = 10m(-)9th). ATOM CENTER, the nucleus is occupied by protons and neutrons of similar mass. This is the mass of the atom, the volume is made by the cloud of electrons. PROTONS, have a positive charge, the number of protons equal the atomic number, hydrogen has one proton. NEUTRONS, have no charge. ELECTRONS, have a negative charge orbiting the nucleus, electrons and protons are equal in quantity, so there equal in (+-) charges. ATOMIC NUMBER, the quantity of protons in the nucleus. ATOMIC WEIGHT, is comparing the atoms of each element to the hydrogen atom. ISOTOPES, the neutron quantity changes the atomic weight of atoms with similar atomic numbers, so each element has isotopes and different atomic weights, all based on the weight of one carbon isotope. SHELLS, electrons orbit on seven tracts or shells, lettered K to Q outward from the nucleus. Each shell has a limit to the number of electrons in it, 2 for K, 32 for Q. Hydrogen has one nuclear proton and one electron in K shell, Lithium, with three protons, has a full K shell and one electron in the L shell. INERT GASES, will not combine with other elements, they have 8 electrons in Q shell, other elements are considered stable if they can attain a similar outer shell content. Atoms trade electrons with other atoms so their electron cloud can become identical with inert gases, the nearest inert gas. IONS, This trading leads to atoms becoming electrically charged and known as ions, gain an electron, gain a negative charge. Lose an electron and gain a positive charge, remember, electrons are negative. ANIONS, are negatively charged ions, they have gained one (-) electron. CATIONS, are positively charged ions, they have lost one (-) electron. VALENCE, the "hook" to hold another "hook", hydrogen has a valence of one, it can hook up with another atom with one valence. Valence is the chemical binding power +/-. VALENCY, the process of gaining or losing electrons. (Na) sodium, has one more electron than the nearest inert gas, neon (Ne), it's written Na+ to show it's a cation. Fluorine (F) has one less and is written F, meaning it's anion. Valency is the number of electrons an atom will gain or lose to attain the configuration of the most similar inert gas. MONO VALENT, ions Na+ and F- are both charged by one electron off being stable and inert. DIVALENT, magnesium has two more electrons than neon, the closest inert gas. By losing both it becomes a divalent cation, written Mg2+. ATOMIC STRUCTURE, (C) carbon and (Si) silicon have atomic structures midway between two inert gases. Carbon can either gain four electrons to become C4- and resemble neon or lose four electrons to become C4+ resembling helium. REPULSION, The electrical charge of protons keeps other protons away by mutual repulsion, they have similar spinning directions. When two atoms link, the interaction is by the outermost electrons. There's three ways to do this. IONIC BONDING, is two elements forming ions of equal and opposite valency, the spare electron of one atom separate to fill a vacancy in the outer shell of another atom. The pair of ions are held together electrically. Groups of ions can be linked this way, a divalent cation can link with two univalent anions. COVALENT BONDING, shares electrons, two or more atoms coming close together share, and both or all, have eight in their outer shell. Solids containing ionic or covalent bonds are rigid. METALLIC BOND, metals pack the ions closely together and leave the outermost electrons free to move independently. This lets them be hammered thin, form wire and conduct electricity. COMPOUNDS. When the linked atoms are of different elements, the result is a chemical compound. COMPLEX, more than one compound. COMPLEX IONS. In many minerals, subgroups of atoms are bonded in a covalent way forming complex ions or radicals. A common example is the tetrahedral arrangement of four oxygen atoms around a single silicon atom, to give a complex anion, written (SiO4)4-. ELECTRICALLY BALANCED COMPOUND, the complex anion (SiO4)4- must be bonded to a cation or cations with a total valency of four, to get an electrically balanced compound. Two atoms of the divalent metal Mg would fit and the mineral forsterite has the composition (Mg2)4+(SiO4)4-, or it could be written Mg2(Si04)4-. MOLECULES. Heating a mineral causes the atoms to vibrate faster, breaking the bonds between them. In this way the independent groups in steam, called molecules, move freely. Share electrons, be a molecule. AMORPHOUS. Substances that do not have the atomic order of crystals are amorphous, they don't have the directional properties of crystal. Opal, natural glass, and flint are amorphous aggregates. ION SHAPE. Crystals are made up of ions having the shape of spheres, these fit together leaving a space in the center. Three oranges and a pea. The carbonate ion has this shape, a pyramid with three sides and a bottom. The common complex ion (CO3)2- is carbonate. TETRAHEDRON. Four oranges and a grape would make a Tetrahedron, the shape of the silicate anion (SiO4)4-. OCTAHEDRON, is two pyramids joined base to base. CUBES, have all equal sized ions, four polyhedra pyramids equal a cube, as in coordination polyhedra and close packing. POLYMORPHS, are the structures possible by a compound, Si02 makes five different structures. Diamond and graphite are polymorphs of carbon, graphite is two dimensional, flat and weak. The diamond is a three dimensional latrahedrally-oriented covalent bond, strong and possibly twinned. TWINNED, two crystals joined inside, interpenetrating each other. ISOMORPHOUS minerals with identical crystal structure. SOLID SOLUTION. Olivines are groups of minerals, magnesium (Mg2SiO4) and iron (Fe,2SiO4) are forsterite and fayolite, melted together. If the olivine contains 75% forsterite it's written like this, (Mg0.75Fe0.25)2SiO4.
COLOR WHEEL IN ELEMENTS AND CRYSTAL
Three examples are; 1, lead, no other element dries as fast or is as opaque. 2, Cobalt natural, (cobalt aluminate blue spinel is just another pre-made color for you to buy), and 3, antimony Naples yellow, probably the artist's most favored color before Ostwald and the ASTM's intervention. They matched the dried color with new cheaper elements without regard to its natural characteristics. Rubins would have had them flogged if they tried that while he was alive. Today there just aren't enough artists that know about the differences. We need a paint manufacture that cares! So, simplifying your choices is the name of the game here, what colors and what characteristics are most needed to complete a full color painting. Use the transparent pigments that are available and be selective in choosing the opaque ones. There are twelve to thirty six colors in my basic color wheels, it's mine because no one else ever put it in print in this order, I want it to be yours. This is original concept work here. I start with yellow at the top and "Read Red Right," the three R's of color. Play the wheel like a typewriter. There are only twelve colors to remember, forward, backward and centering across the middle. Across the middle is what makes the Real Color Wheel different from all the rest. It matches the pigment color wheel with the RGB/YMCR color wheels so they both work at there best. In a twelve color wheel, Yellow is represented by "Y," Magenta by "M," and Cyan "C." A pure unadulterated yellow would be YYYY, half yellow and half magenta would be YYMM, or the color red, each color has four characters. Orange is YYYM. Scarlet-crimson is YMMM. The sixth color away from any color is halfway around a twelve-color wheel making it the opposite, or the complementary color. The opposite of YYYY is MMCC, Ultramarine Blue. HERE ARE THE SYMBOLS OF ALL TWELVE SUBTRACTIVE PIGMENT COLORS YYYY=Yellow, YYYM=Orange, YYMM=Red, YMMM=Scarlet-Crimson, MMMM=Magenta,
MMMC=Purple, MMCC=Ultramarine, MCCC=Azure, CCCC=Cyan, CCCY=Turquoise,
CCYY=Green, CYYY=Yellow-green. There is no pre-made transparent pigment paint for the color MCCC, Azure, Cobalt Blue is a close color but opaque. It matches the ancient color of Azurite. Cu3(C03)2(0H)2, a copper idiochromatic color. Azure can easily be made with copper phthalocyanine blue and a good ultramarine blue or a little cobalt violet (magenta). In fact with a cobalt violet, (the cool magenta), you won't need ultramarine blue, you can make it. One way or the other, Azure is a beautiful color that nature uses often. Each element can only make its own range and texture of colors. No other element has its similar unique capabilities. Modifying the element can make its opposite or complementary color, as the cuprite crystal of copper and the sediment of copper do naturally. By explaining minerals and crystals, and the elements that make and color them, you will understand the real color wheel and know the pigments that make it go 'round. This color wheel joins the pigment and the light color wheel together as one, and agrees with the nature of your eyes "afterimage." To make the light color wheel match the pigment color wheel, replace
Yellow's neutral dark (which has a Green tinge, with Burnt Umber), (that's the
Red's normal dark). Than change Cyan's centering dark to Ultramarine Blues dark.
Now all the colors in both palettes will have working oppositions.
CRYSTALS, LIGHT AND COLOR TERMS ALEXANDRITE EFFECT, The chrysoberyl alexandrite is red in candle light and green in daylight. ALLOCHROMATIC, minerals colored by a foreign element are called allochromatic. Ruby is an aluminum oxide colored by chromium, so it's allochromatic foreign and not written in the symbol AL203. IDIOCHROMATIC, minerals colored by elements which are a regular part of the chemical composition. The peridot crystal is in the olivine category, it's magnesium, iron, silicate. The green comes from iron, making this an idiochromatic home color (not foreign) crystal. ANALOGOUS, colors are side by side on the color wheel rim. INCIDENT ANGLE, lights entering angle. REFRACTIVE INDEX, when light enters a cube or amorphous structure the incident ray is slowed by the atoms inside. The ratio of the speed of light in a vacuum compared to the speed in the structure is called the refractive index. In crystals other than the cube, the light splits in different directions at different speeds. REFRACTION, The deviation of a ray of light upon entering a transparent medium, is refraction away from the normal. The emerging ray is separated into the colors of the spectrum. Red refracts most, violet and magenta the least. DOUBLE REFRACTION, an incident ray enters and splits, each ray traveling at a different speed. DISPERSION, diamonds, glass and water give dispersion of light with the proper incident and reflecting angles, thus splitting up white light into its spectrum colors. The water droplets in a rainbow are at the proper angle of refraction to give each color seen by you. Each person sees their own rainbow. The axis from the sun through your eye to the center of the rainbow's circle is the basis for the angle of an incidence you see as the rainbow's refracted light. Any medium in which the high-frequency light travels more slowly than the lower frequencies are called dispersive. DIFFRACTION, diffraction is light bending around the edges of a mass. ISOTROPIC, crystals in which light travels in all directions at the same velocity. INTERFERENCE COLORS, a thin film of soap or oil will change the direction of the incident ray laterally and cause the prism effect, as the thickness changes the color's change. LUMINESCENCE or FLUORESCENCE, is stimulated by radiation. PHOSPHORESCENCE, persisting luminescence after stimulation. PLEOCHROISM, the change of color when viewed from different directions. CRYSTAL CHROMATE (COLOR) ELEMENTS THERE ARE 92 NATURAL ELEMENTS. EIGHT ELEMENTS MAKE UP 99% OF THE EARTH'S CRUST. On the ELEMENT PERIODIC TABLE, #22 through #30 are the coloring elements that give color to crystal compounds. Elements color in harmonies. Analogous, is side by side around the rim of the color wheel. Centering, means moving from the high chroma rim to the center's natural dark position. Complementary colors are opposite colors, 180 degrees apart, triadic colors form an equilateral triangle, they're 120 degrees between colors. Split-complementary colors are on each side of the opposite color, making a "Y". Aluminum- Easily displaced, makes yellow and sometimes blue. Arsenic- Yellow, orange red. Cadmium- Green, yellow-green, yellow, orange, red to deep red, a substratum dye. Cadmium is not a solid pigment. Chromium- Analogous system. Green (emerald), Yellow-green, Yellow, Orange, and Red (ruby). Cobalt- Oxides and native, Magenta (MMMM) and cool magenta (MMM1/2C), natural or calcined to Ult.Blue (MMCC). Copper- Green (CCYY) in malachite, Cyan-green (CCCY) in turquoise, cyan-blue (CCCM) in azurite, all analogous colors of the copper element. Iron- Yellow (YYYY) in sapphire, Blue (MMCC)in spinel, opposite colors. Green (CCYY) and Red (YYMM) in sphalerite. Coppreas, green vitriol, or ferrous sulfate. Lead- White,Yellow, Orange, Red. Manganese- Light Pink-Orange (YYMM) in spessartine and rhodochrosite. Nickel- Yellow-Green (YYYC) in chrysoprase. Titanium- White pigment, titanium dioxide. Titanium is a centering element that goes from Yellow to Orange, then Red to Brown and Brown to Blue in the Rutile crystal. It is the only element that can cross over the center dark, reaching it's complementary color Ultramarine Blue. The Real Color Wheel uses this pattern to darken Yellow instead of using Black as in RGB color wheel. Vanadium- Green (CCYY) in beryl, Yellow (YYYY), Brown (YYMMCC). Zinc- Yellow-green in sphalerite.
COLOR PRODUCING ELEMENTS, SYM., NO., S/G, DESCRIPTION SPECIFIC GRAVITY, (S/G) The ratio of size to weight by water displacement,
Corundum SG=4, four times the weight of the same volume of water. Archimedes'
Principle, SG= A/A-W. ELEMENT SYM No. S/G Aluminum Al 13 2.7 metallic, ore bauxite. Antimony Sb 51 4.6 brittle metallic, ore stibnite. Arsenic As 33 poisonous, pentavalent As+5. Astatine At 85 rare element, halogen family, unstable. Barium Ba 56 3.5 malleable, active, divalent metal, compounds in barite. Beryllium Be 4 1.8 hard, light, divalent, steel-gray metallic element, idiochromatic primary and secondary colors. Boron B 5 ore of Borax. Bromine Br 35 3.1 liquid, dark-red fuming, resembling chlorine and iodine. Cadmium Cd 48 8.6 divalent metallic element, allied to zinc, Pigment in 1842. Calcium Ca 20 divalent metal, compounds limestone, chalk, gypsum. Carbon C 6 Diamond and graphite are organic, polymorphs of carbon, graphite's two dimensional atomic structure is flat, and is soft graphite, black, The three- dimensional tetrahedrally-oriented covalent bond of diamonds is strong, hard and clear, the opposite of carbon. Diamonds can receive the full range of colors from other elements, they are allochromatic. Chlorine Cl 17 Gaseous, combined in salt. Chromium Cr 24 Brittle metallic, source of red in allochromatic compounds. Cobalt Co 27 Metallic silver pink element. Copper Cu 29 8.92 Malleable, red-brown metal element. Fluorine F 9 Non-metallic corrosive pale yellow gas, combined in fluorite. Gold Au 19.3 Yellow malleable metal. Hydrogen H 1 Colorless inflammable gas, lightest element. Iodine I 53 4.93 dark-gray crystalline solid, heats to a dense violet vapor. Iron Fe 26 7.86 Malleable metallic, silver element. A full spectrum element. Lead Pb 82 11.34 Malleable, blue-gray metal. Lithium Li 03 .53 Silver-white. Soft, lightest metallic. Magnesium Mg 12 1.74 Silver-white metallic, burns white hot. Manganese Mn 25 7.2 Brittle, gray-white element, allochromatic. Mercury Hg 80 13.55 Fluid silver-white metallic element. Molybdenum Mo 42 10.2 Hard, silver-white high melting, metalloid. Nickel Ni 28 8.9 Hard silver-white, malleable, allochromatic element. Oxygen O 8 Colorless gas, converts elements into oxide compounds. Phosphorus P 15 1.82 Solid non-metallic element in two allotropic forms, yellow, poisonous, inflammable and luminous. Red, now rare, is less potent, (SG) 2.20. Platinum Pt 78 2.5 Malleable, gray metallic element. Potassium K 19 .86 Silver-white metallic, oxidizes rapidly. Selenium Se 34 4.8 Gray, non-metallic allotropic element, resembles sulfur. Selenium Se 34 4.5 Red, similar to gray. Silicon Si 14 2.4 Non-metallic element, amorphous and crystalline forms. Silver Ag 47 10.5 Malleable silver element. Sodium Na 11 .97 Soft silver-white metallic element, oxidizes in moist air. Strontium Sr 38 2.6 Bivalent metallic element found only in a combined state. Sulfur S 16 2.07 Nonmetallic solid element, used to form sulfates as white lead pigment, and sulfides, burns blue. Titanium Ti 22 4.5 Dark gray powdered metallic element Tin Sn 50 7.31 Malleable, low melting silver colored metal. Tungsten W 74 19.3 Bright-gray, metallic. Uranium U 92 18.7 White, radioactive, metallic. Vanadium V 23 5.96 Gray powder metallic, rare. Zirconium Zr 40 6.4 Metallic, resembles titanium. Zinc Zn 30 7,14 Blue-white metallic element.
MINERALS AND ELEMENTS IN CRYSTAL COMPOUNDS WITH COLOR CHART NUMBERS GO TO THE CRYSTAL COLOR CHART, Brittle crystals make the best pigments. Hard oxides of metal are inert pigments. Idiochromatic is an internal element coloring the crystal. C, (Hardness) H10, Diamonds can receive the full range of colors from other elements, they are allochromatic. This section describes the color and properties each element adds. 01, LIDDICOATITE, a six sided continuous crystal, clear and colored by iron. The colors of magenta and green are separated in sections and graduated from one end to the other in each crystal, a natural color opposition. All mineral opposition's match my pigment and light color wheel.
CHALCOPYRITE, CuFeS2, (Hardness), H3.5, (specific gravity) SG-4.2. Ore of copper, tetragonal crystal system. It occurs on crystals of galena and has a brassy yellow opaque, metallic color. Idiochromatic colors of iron. 03, SPHALERITE, Zn,S, H3.5, SG-3.9, zinc blend, ore of zinc, cubic system, idiochromatic colors. ZINC, yellow-green, standard color #11, (CYYY), transparent. IRON, Idiochromatic colors from, yellow, tan, brown, black, orange and red. High dispersion showing the spectrum like a diamond. CHROMIUM, crimson and dark green. Transparent to opaque. 04, CINNABAR, HgS, H2, SG-8.09, sulphide of mercury ore. MERCURY, red, standard color #3, (YYMM), opaque, vermilion-red to brown is the color scale for the masses, and transparent scarlet is the color for crystals. Cinnabar has internal coloring, idiochromatic coloring, because the color comes from the element that's crystallizing. In this case mercury, the liquid metal. The amorphous mass is crushed and used as the pigment vermilion, it's a heavy and a fast drier. Precious and rare. 05, GALENA PbS, H2, SG-7.6, ore of lead, sulfide, cubic crystals often twinning. Oxides are white lead pigment. REALGAR, AsS, H1.5, SG-3.56, monoclinic crystal system. ARSENIC sulphide, red standard color, #3, (YYMM), transparent to opaque. Realgar was crushed as a pigment by the ancients as the first red. It's an idiochromatic crystal pigment. 07, ORPIMENT,As23, H1.5, SG-3.4, monoclinic, transparent to opaque. ARSENIC di-sulphide, orange standard color, #2, (YYYM), yellow to orange pigments, crushed, idiochromatic pigment. 08, STIBNITE, Sb23, H2, SG-4.6, ore of antimony, orthorhombic crystal system, lead-gray metallic. Antimony oxide native, is Naples yellow pigment. PYRITE, FeS2, H6, SG-5, cubic, metallic
brass-yellow. 10, PROUSTITE, Ag3AsS3, H2, SG-5.6, trigonal, transparent. SILVER and ARSENIC, crimson color at it's best. It tarnishes because of the silver content, idiochromatic, silver added the deep magenta to the red of arsenic. 11, PYRARGYRITE, Ag3SbS3, H2.5, SG-5.85, ore of silver, trigonal. SILVER AND ANTIMONY, a deep warmer crimson than proustite. Idiochromatic, deep crimson, transparent, dark in mass.
12, CUPRITE,Cu20, H3.5, SG-6.14, cubic, crystals found on copper. COPPER, crimson, standard color #4, (YMMM), transparent. Crimson cuprite crystals are opposite in color to the turquoise colored copper sediment, chalcocite. Idiochromatic. 13, CHRYSOBERLE, BeAl2O4, H8+, SG-3.74, orthorhombic crystal. BERYLLIUM, standard yellow centering to brown crystal. ALUMINUM, yellow is aluminum's home base, but color isn't really it's strong suite, it's the light tricks. The "cat's eye" gem, it has an opposite color line of light inside, a pale ultramarine blue. Instead of just giving color, aluminum has an array of light tricks to perform with. 14, SPINEL, MgA1204, H8+, SG-3.6, cubic octahedra, idiochromatic. MANGANESE, deep transparent red pigment color that lightens to Cadmium Red opaque. The Ruby is Cadmium Red transparent. Idiochromatic. ALUMINUM, adds yellow sometimes, enhances other elements. CHROMIUM, allochromatic chromium, which gave the red to rubies gives spinel a real kick in the red mass dark, and it never leaves the red spectrum color. COBALT, bright blue, with allochromatic cobalt, it does well here in aluminum's opposite second home. 15, ZINCITE, (Zn,Mn)O, H4, SG-5.6, Native zinc oxide, ore of zinc, MANGANESE, yellow-orange to deep red, idiochromatic, it could be a pigment, rare, brittle, and opaque. 15, ZINC SILIATE, is found in New Jersey, at the Franklin Mine. ZINC, yellow, yellow-green and green transparent, and opaque white, opaque yellow, orange, red, brown and black. Idiochromatic zinc makes all the colors except those in the cyan spectrum. 16, CORUNDUM, Al2O< sub>3, H9 STANDARD MOHS', SG-3.99, trigonal system, transparent. CHROMIUM added for red and green, allochromatic. IRON, blue, cyan, dark-green, yellow, orange, red and magenta. All transparent, all brittle, and all expensive as crushed pigments. Ruby is the standard transparent red, #3, (YYMM). It's opposition color mixing to dark neutral is Thalo Blue,(Cyan). In pigments, Thalo Blue Transparent mixes dark with Cadmium Red Opaque also. The other oppositions in paint are acrylic Thalo Green and Acra Violet by Liquitex. Burnt Umber (for yellow) and Opaque or Transparent Ultramarine Blue. All covered later in the painting section. Sapphire blue is the standard transparent blue, #7. (MMCC). Sapphire yellow is the standard transparent yellow, #1, (YYYY). Sapphire pink deep is the standard magenta, #5, (MMMM). ALUMINUM is just one surprise after another, here it is, the softest metal element making the hardest hydroxide, only the softer carbon element makes a harder crystal, the diamond is H10 STANDARD MOHS'. 17, QUARTZ, SiO2, H7 STANDARD MOHS', SG-2.65 trigonal, clear crystal with allochromatic colors. MANGANESE, standard purple, #6, (MMMC), transparent, amethyst crystals are from magenta to purple. IRON, rose quartz is a light magenta. Citrine is from yellow to orange, centering dark, through brown. Iron also gives black (deep-red) to onyx, and red and orange in fire agate. A second verity of quartz is mass and opaque, the aggregate crystals are too small to see. COPPER, green to cyan, chalcedonies, jasper, and red carnelian, two opposing colors. NICKEL, yellow-green standard color, #12, (CTTT), chrysoprase. Quartz has a complete range of colors except for ultramarine blue. All are allochromatic with foreign elements, giving color to the crystal. Brittle, and easy to crush into pigments. 18, OPAL, Si02+NH2O, H6.5, SG-2.1, amorphous crystal, diffraction
from spheroids give color. Transparent, translucent and opaque in different
crystals. 19, RUTILE, TiO2, H6.5, SG-4,23, tetragonal. TITANIUM, red to brown and yellow to brown, colors centering to neutral dark. Just on the other side of the darkest center in the coloring wheel, brown is mixing with ultramarine blue and getting cooler, some of that blue color shows up. Titanium is the only element that can cross over the dark center. Idiochromatic. Transparent, translucent and opaque in the same crystal. Purple rutile is also found in the Alps, diochromatic. 20, ANATASE, Tio2 H5.5, SG-3.9, tetragonal, metallic luster. TITANIUM, yellow to yellow-brown or red to red-brown, brown to a neutral dark by continued mixing with the opposite spectrum colors, ultramarine, azure and cyan. All idiochromatic with titanium. 21, CASSITERITE, Sn02, H6, SG-6.9, ore of tin, tetragonal, metallic. The crystals are clear, yellow, red-brown or black. A one trick pony. This is the yellow pigments natural trip to neutral dark. 22, HEMATITE, Fe2O3r H5r SG-5.26, ore of iron, trigonal reniform masses, Cadmium Red streak, metallic.
23, HALITE, NaC1, H2.5, SG-2.2, salt is a cubic crystal that may show orange, spectrum purple #6 (MMMC) or blue coloration. It is brittle, soluble, and transparent. 24, FLUORITE, CaF2, H4, SG-3.18, cubic system. Fluorite is allochromatic, it accepts a range of eleven colors, there is no cyan color. Fluorite is also a fluorescent, green to violet, Translucent. 25, CUMENGEITE, .Pb21Cu2OC142< /sub>(OH)40, H2.5, SG=4.6, cubic or tetragonal, two different crystals, depending on the majority mineral element. LEAD, standard ultramarine blue color, #7, (MMCC), opaque, idiochromatic. Cumengeite. COPPER, standard cyan color, #9, (CCCC), opaque, idiochromatic. Boleite crystals.
26, MALACHITE, Cu2(C03)(0H)2, H4, SG-3.6, mass or monoclinic systems. COPPER, green standard color, #11,(CCYY), opaque, crushed pigment, idiochromatic. 27, AZURITE, Cu3(CO3)2(0H)2, H3.5, SG-3.7, monoclinic. COPPER, Standard Azure color, #8, (MCCC). Cyan-blue transparent to opaque, shows blue in mass. This was a popular ancient pigment color, crushed, rare. 28, CALCITE, CaCO3, H3 STANDARDMOHS', SG-2.7, trigonal crystals, also aggregate and mass, colorless transparent to translucent. THE COMPLETE PRIMARY PIGMENT TRIAD REAL COLOR WHEEL IN CRYSTAL. IRON, Yellow allochromatic. COPPER, "Iceland Spar", Cyan, allochromatic, polarizing filter. COBALT, "Sphaeroc Cobaltite", standard Magenta Cool,#5, (MMMM),
allochromatic. 29, ARAGONITE, CaCo3, H3, SC-2.94, ortharhombic system, phosphorescent, clear. 30, DOLOMITE, CaMg(Cu3)2, H3.5, SG-2.85, trigonal, translucent clear, double refraction 31, WITHERITE, BaCO3, H3.5, SG-4.3, orthorhombic crystal system with twinned hexagonal pyramids, that's like the four sided pyramid the Egyptian's were so fond of. Light-translucent, colorless or centering yellow-green to brown, allochromatic. Ore source of barium, found with lead. Pigment extender. 32, SMITHSONITE, ZnCO3, H4, SG-4.4, trigonal crystal system. ZINC, translucent green, Idiochromatic, pale. 33, AURICHALCITE, (Zn,Cu)5(Cu3)2(0H)6, H1, SG-3.64, orthorhombic. White opaque. ZINC AND COPPER, turquoise-green outside, white inside, opaque, crystal forming fragile hollow balls, idiochromatic. 34, CERUSSITE, PbC03, H3.5, SG-6.5, orthorhombic of many forms, clear transparent, brittle lead and carbon. 35, RHODOCHROSITE, MnCO3, H3.5, SG-3.7, scalenohedral crystals in the trigonal system, opaque and transparent. MANGANESE, red standard color, #3, (YYMM), idiochromatic. SIDERITE, FeCO3, H4.0, SG-3.9, trigonal, rare gemstones. IRON, yellow-green to brown, centering colors in different crystals,
transparent or translucent. This crystal is the cool side of the Yellow scale to
neutral dark.
37, DIOPTASE, CuSiO2(OH)2, H5, SG-3.3, trigonal, opaque. COPPER, Dark-turquoise standard color, #10,(CCCY), idiochromatic, brittle. An ideal native pigment. 38, CHRYSOCOLLA, (Cu,Al)2H2Si2O5( OH)4.nH2O, H2, (with quartz H7), SG-2,6, monoclinic aggregate, idiochromatic copper. COPPER, turquoise standard color, #10, (CCCY), pale opaque. Rare crushed opaque pigment, replaced by man made Egyptian frit for a huge market by the Phoenicians. 39, PHENAKITE, Be2SiO4, H7.5, SG-3, trigonal rhombohedral crystals, clear, allochromatic, triadic. NOTICE: CLEAR IS OPPOSITE OPAQUE. WHITE IS A TRANSLUCENT TO OPAQUE NOT CLEAR. CLEAR HAS NO WHITE IN IT. BERYLLIUM, magenta, cyan, yellow, all pale and transparent. ANOTHER ALL COLOR CRYSTAL. 40, WOLLASTONITE, CaSiO3, H5, SG-3, triclinic, tabular or masses. White opaque, orange fluorescence. 41, DIOPSIDE, CaMgSi2O6, H6, monoclinic. MAGNESIUM, yellow-green or purple (opposites), internal coloring. CHROMIUM, dark green. Brittle translucent, foreign coloring. 42, SPHENE, CaTiSiO5, H5.5, SG-3.5, monoclinic wedged shaped crystals and masses. TITANIUM, yellow-green or yellow, to brown with neutral dark areas, in a transparent crystal. Centering yellow's to neutral dark, through warm brown, is a common trait observed with titanium and iron. This is the way my coloring wheel works with yellow going to Brown as the neutral dark instead of Black. To reach Black, one would need a Transparent Yellow pigment that hasn't been around for a hundred years (1900). The outlawed Indian Yellow, from cow urine, from mango leaf feed cows in India. This would mix with a Transparent Ultramarine Blue to a neutral dark. We artists have been deprived. Idiochromatic. CHROMIUM, green, rare crystal, allochromatic. 43, ZIRCON, ZrSiO4, H7, SG-4.6, tetragonal system ends with a four sided pyramid, transparent. Clear to red-brown opaque. ZIRCONIUM, full spectrum of pale colors, additional heat makes yellow and blue crystals. Brittle, idiochromatic, the transparent crystals are doubly refractive and
rival the diamond in dispersion. 44, SERANDITE, Na(Mg2+,Ca)2Si3O8(0H), H4.5, SG-3.5, triclinic. MANGANESE, red-orange, pale, opaque. A weak manganese color with two electrons gone, idiochromatic. 45, OLIVINE, (Mg,Fe)2SiO4, H7, SG-3.3, orthorhombic crystals. MANGANESE, red-orange opaque, shows up as brown when mixed with yellow-green, like the light color wheel, idiochromatic. IRON, yellow-green, transparent, with two minerals, iron making a transparent yellow-green and manganese making an opaque split-analogous red-orange, what you get is a transparent yellow-green going to opaque brown in the same crystal, or anywhere in between, the peridot series. 46, KYANITE, Al2SiO5, H7.5 across 4.5 down, SG-3.7, triclinic. ALUMINUM, white, pale-green to gray, opaque to translucent. Pleochroistic, (changing colors with direction), transparent light green background when perfect, with a "blue light" stripe, translucent. The aluminum element plays tricks with light that no other element can. 47, SPODUMENE, Li AlSi2O6, H7, SG-3, monoclinic, transparent, ore of Lithium. LITHIUM, yellow-green, yellow, orange, red, and a Cool Magenta Standard crystal called "Lilac Kunzite". Clear to pale translucent, idiochromatic. Red-orange phosphorescent, the opposite of the missing cyan. ALUMINUM, yellow-orange, enhances iron and chromium. IRON, warm green, allochromatic. CHROMIUM, cool green, allochromatic, works well with aluminum, as in the Corundum compound (Ruby). 48, LEUCITE, KAlSi2O6, H5.5, SG-2.5, tetragonal, opaque, white. ALUMINUM, very pale yellow-orange crystal. The aluminum spin on this compound is interference colors on the cut face, like an oil film on water, with orange fluorescence in long-wave ultraviolet light and blue under X-rays. 49, ORTHOCLASE, KAlSi308, H6 STANDARD MOHS', SG-2.5, monoclinic. ALUMINUM, pale yellow-orange opaque crystals. The transparent yellow gem (moonstone), has internal light diffusion, another light trick by the inclusion of aluminum. 50, BERYL, Be3Al 2(Si03)6, H7.5,SG-2.7, hexagonal crystal system. BERYLLIUM element in Beryl crystal, cyan, yellow, idiochromatic. ALUMINUM, yellow-orange, idiochromatic, IRON, green, yellow-green, yellow, orange, red, scarlet, magenta purple, blue, azure, cyan, turquoise, iron at it's best will display the whole spectrum in Beryl. Allochromatic. CHROMIUM, green standard, #11, (CCYY) and red, allochromatic. MANGANESE, red, allochromatic. Double elements in yellow #1,(YYYY), cyan #9,(CCCC), red #3, (YYMM), and green #11,(CCYY), all standard colors. Yellow (heliodor), magenta (morganite), cyan (aquamarine) and green (emerald). ALUMINUM in beryl excepts a lot of foreign chromates, just as it does in
the corundum and spodumene compounds, Here an aluminum light trick is found in
the aquamarine crystal, light cyan is seen from one direction and deep blue from
a 90 degree off angle. EUCLASE, BeASi04(0H), H6.7, SG-3, monoclinic system. BERYLLIUM, standard color cyan, #9,(CCCC), yellow to cyan, idiochromatic, transparent. 52, GROSSULAR, Ca3Al2(SiO4)3, H7, SG-3.5, cubic, garnet group. ALUMINUM, standard color orange, #2,(YYYM), yellow to orange, centering to brown, idiochromatic, transparent, CHROMIUM, green, allochromatic. 53, LABRACORITE, NaAlSi3O8-CaAl2Si2O< sub>8, H6, SG-2.5, sodium-aluminum series of plagioclase feldspars. ALUMINUM, blue to cyan color plus an unusual effect called schiller, the inside glow of cyan color moves with you, playing off included lamellae minerals. Idiochromatic, opaque. IRON, yellow to red, the allochromatic intrusion of hematite reverses the spectrum to show it's opposite colors. 54, SPESSARTINE, Mn3Al2(SiO4)3, H7, SG-4, cubic, garnet family. ALUMINUM, yellow-orange, turning red to orange with manganese. MANGANESE, orange to red, transparent, idiochromatic. IRON, red, transparent, allochromatic, adding transparent red until it's a deep transparent red. This is only a 30 degree span of color with no complementary intrusion toward centering. This is the light "almandine garnet". 55, ALMANDINE, Fe3Al2(SiO4)3, H7, SG-4, cubic, transparent, Garnet. ALUMINUM, yellow-orange, added to the red of iron, idiochromatic. IRON, red, idiochromatic, transparent. CHROMIUM, red, transparent, allochromatic. These two elements together make a transparent red and transparent red deeply saturated. This red is so deep and spectrum dark it has to be very thinly sliced to see any color at all. "Deeply saturated" means a transparent to opaque color skipping translucent. 56, EPIDOTE. Ca2( Al,Fe3+)3(SiO4)3 (OH), H7, SG-3.4, monoclinic. ALUMINUM, yellow-orange, transparent, idiochromatic, pleochroistic directional change of color. IRON, yellow-green Epidote has a unique coloring system, combining split analogous colors yellow-green and orange, through brown to a warm neutral dark transparent color. 57, VESUVIANITE, Ca10Mg2Al4(SiO4)5 Si207)2(OH)4, H7, SG-3.3, MAGNESIUM, wants to go dark and opaque, or transparent, either way, it does it with flair. ALUMINUM, yellow, transparent, standard #1(YYYY), and blue, transparent, standard #7 (MMCC), centering to neutral. Crystals in the tetragonal system, some masses in the orange or azure range. By centering yellow to brown, the three analogous colors of this gem, yellow-green, yellow and orange go to brown. On the other side of this analogous range is blue, blue crystals were found in Norway. Idiochromatic. CHROMIUM, green, allochromatic. 58, APOPHYLLITE, KCa4Si8O2O(F,OH)8H2O, H4.5, SG-2. White to pale cool green, opaque, idiochromatic, non-metallic mineral. 59, TOPAZ, Al2(F,OH)2SiO4, H8 STANDARD MOHS', SG-3.53, orthorhombic crystal system. ALUMINUM, yellow, mostly orange, red and magenta, some azure crystals come from Zimbabwe. Idiochromatic colors of aluminum under high pressure. 60, ELBAITE, Na(Li,Al)3Al6BO3)3Si618(OH)4, H7.25, SG-3.05, trigonal, tourmaline group, transparent, pyroelectricity w/heat. IRON, green, red, and pink Magenta, brown. LITHIUM, yellow, magenta, idiochromatic. ALUMINUM, yellow, blue, idiochromatic. MANGANESE, orange and red, allochromatic. COPPER, green, cyan, allochromatic. Many Elbaite crystals show more than one color in a clear rainbow ordered spectrum. 61, LAPIS LAZULI, H5-6, SG-2.8, or higher if pyrite is included. This is the Standard Color for ultramarine blue opaque, #7, (MMCC), an ancient pigment. A rock of many compounds. Lazurite, a sodium aluminum silicate with sulfide, deep blue crystals. Hauyne, Sodalite, (a sodium aluminum silicate with sodium chloride that occurs in crystals and masses), and Nosean. Lapis lazuli is a contact metamorphic mineral found in limestone and granite, the best is found in Afghanistan, from ancient times until today.
62, TURQUOISE, CuAl6(PO4)4(OH)8.4 -5H2O, H5.5, SG-2.7, triclinic. ALUMINUM, transparent. COPPER, turquoise standard color, #10, (CCCY), transparent. Transparent turquoise crystals are rare, it's usually found in opaque masses, idiochromatic. 63, WAVELLITE,Al3 (PO4) 2(OH,F)3.5H2O, H3.5, SG-2.36, orthorhombic crystal system, acicular, like the needles of Rutile suspended in clear crystal, only these needle crystals form a dense rocklike mass. ALUMINUM, yellow, brown, blue, centering standard, transparent. Aluminum really does it big this time, each needle starts at the center radiating outward, concentrical ringed bands form around the center, each band ending as a cleavage line, twenty or so bands per complete needle crystal. The band itself can change from clear transparent to opaque white while in the center, the second band range is transparent yellow crystal, then brown, than blue. From brown to blue, the crystal can change into an aggregate and lose it's crystal properties. THIS MINERAL PHOSPHATE SHOWS THE CENTERING OF YELLOW TO BROWN AND BROWN TO BLUE, LIKE MY REAL COLOR WHEEL FOR PIGMENTS. 64, BRAZILIANITE, NaAl3(PO4)2(0H)4, H5.5, SG-2.9, monoclinic. ALUMINUM, yellow, cool, transparent, idiochromatic. 65, AUTUNITE, Ca(UO2)2(PO4)2.10-12H2O, H2, SG-3.1, tetragonal. URANIUM, yellow, translucent, Idiochromatic uranium, warm to cool yellow, pigment in the 1940s, fluorescent yellow. 66, VIVIANITE, Fe3 (P04).8H20, Hardness 1.5, SG-2.68, monoclinic. IRON, standard color for cyan, #9,(CCCC), transparent, idiochromatic. Cyan to green crystals. Laminae fibrous, long thin radiated crystals, transparent clear when first mined, turning cyan with light exposure. 67, LEGRANDITE, Zn2(AsO4)(OH),H20 H5, SG-4, monoclinic family. ZINC, yellow, cool, transparent, idiochromatic. ARSENIC, standard yellow, #1, (YYYY), transparent, idiochromatic. Bright yellow idiochromatic, with zinc and arsenic. Arsenic taking it to the warm side in the luminous shadows. Probably the strongest yellow color in crystal form. 68, FLUORAPATITE, Ca5(P04)3F, H5 STANDARD MOHS', SG-3.2, apatite family, hexagonal system, transparent. Yellow, yellow-green, green, cyan, blue, purple and a violet close to magenta, MMM,1/2M-1/2C. Two-thirds of the color wheel without any metallic elements in the compound. 69, PYROMORPHITE, Pb5(P04)3C1, H3., SG-6.5, hexagonal system. LEAD, cool yellow to bright yellow. LEAD MAKES TWO OPPOSITE COLORS, BLUE AND YELLOW, MORE NATURAL PROOF OF MY PIGMENT COLOR WHEEL. Yellow to yellow-green in the shadows, translucent, some times found on azurite, the opposite color. 70, MIMETITE, Pb5(AsO4)3CAsO1, H3.5, SG-7.24 monoclinic, transparent. LEAD, yellow, cool, idiochromatic. ARSENIC, yellow to orange, idiochromatic. Cool yellow, warm yellow and orange analogous colored crystals are found around the world. 71, VANADINITE, Pb5(VO4)3C1, H3, SG-6.8, hexagonal system. VANADIUM, scarlet, transparent, idiochromatic. LEAD, standard red, #3, (YYMM), transparent, idiochromatic. Vanadium, a rare metallic element, compounded with lead makes a red transparent crystal.
72, GYPSUM, CaSO4.2H20, H2 STANDARD MOHS', SG-2.32, monoclinic, white, idiochromatic yellow and green to a centered brown. 73, CELESTINE, SrSO4, H3.5, SG-4, orthorhombic, ore of strontium. STRONTIUM, blue or yellow, idiochromatic, opposites. THIS CRYSTAL IS ONE OF MANY THAT PROVES WITH NATURE; THE REAL COLOR WHEEL HAS THE SAME THIRTY SIX, TWENTY FOUR, TWELVE, SIX AND THREE COLORS, BOTH IN LIGHT AND PIGMENT. 74, BARITE, BaS04, H3.5, SG-4.5, orthorhombic. Ore of barium. BARIUM, yellow to yellow-orange and blue to azure, opposite colors. Transparent to translucent, idiochromatic.
75, CROCOITE, PbCrO4, H2.5, SG-5.99, monoclinic, transparent. LEAD, no color, transparent. CHROMIUM, red, transparent, idiochromatic.
76, WULFENITE, PbM4, H2.75, SG-7, tetragonal. LEAD, no color, transparent. MOLYBDENUM, yellow, orange, red, transparent, idiochromatic.
77, SCHEELITE, CaWO4, H4.5, SG-6, tetragonal, forming octahedra crystals or masses, ore of tungsten. TUNGSTEN, colorless, yellow, orange, brown, transparent, crystals.
Idiochromatic. Tungsten has very unique spectrum of colors, it's a curve to
neutral dark. THIS CRYSTAL HAS THE SAME COLOR PATH AS THE PIGMENT INDIAN YELLOW,
which we no longer have available. Banned in 1899 by England, W/N. 78, WOLFRAMITE, (Fe,Mn)WO4, H4.5, SG-7.5, monoclinic. IRON, red, matching the manganese, iron can be, and can center any color to a neutral dark, red is in the yellow half of the color wheel, and YELLOW CENTERS THROUGH BROWN. Idiochromatic. MANGANESE, red, transparent, idiochromatic, Manganese and iron, two red elements making a very deep red-brown that looks black, found with white and clear quartz. STANDARD-COLORS 12 STANDARD COLORS IN CRYSTAL MINERAL COMPOUNDS THE REAL COLOR WHEEL IN CRYSTAL Click a number to read about the crystal. (1) YELLOW, #13, #16, #50, #57, #67 YELLOW, BROWN, NEUTRAL DARK, BLUE, #63 (3) RED, #4, #6, #16, #35, #50, #71 (7) ULTRA BLUE, #16, #25, #57, #61 (8) AZURE, #27 CRYSTAL-COLOR-CHART MINERAL ELEMENTS IN CRYSTAL BY COLOR, CHART INTERNAL ELEMENT COLORING, IDIOCHROMATIC (+), EXTERNAL ELEMENT COLORING, ALLOCHROMATIC (-) FOREIGN STANDARD PIGMENT COLORS (BOLD) ALUMINUM = Al, ANTIMONY = Sb, ARSENIC = As, BERYLLIUM = Be, BARIUM = Ba, CARBON = C, CHROMIUM = Cr, COBALT = Co, COPPER = Cu, CALCIUM = Ca, FLUORINE = F, IRON = Fe, LEAD = Pb, LITHIUM = Li, SODIUM = Na, MAGNESIUM =Mg, MANGANESE = Mn, MOLYBDENUM = Mo, MERCURY = Hg, NICKEL = Ni, STRONTIUM = Sr, SILVER = Ag, TIN = Sn, TITANIUM = Ti, TUNGSTEN = W, URANIUM = U, VANADIUM = V, ZIRCONIUM = Zr, ZINC = Zn
ALUMINUM = Al, ANTIMONY = Sb, ARSENIC = As, BERYLLIUM = Be, BARIUM = Ba, CARBON = C, CHROMIUM = Cr, COBALT = Co, COPPER = Cu, CALCIUM = Ca, FLUORINE = F, IRON = Fe, LEAD = Pb, LITHIUM = Li, SODIUM = Na, MAGNESIUM =Mg, MANGANESE = Mn, MOLYBDENUM = Mo, MERCURY = Hg, NICKEL = Ni, STRONTIUM = Sr, SILVER = Ag, TIN = Sn, TITANIUM = Ti, TUNGSTEN = W, URANIUM = U, VANADIUM = V, ZIRCONIUM = Zr, ZINC = Zn
ALUMINUM = Al, ANTIMONY = Sb, ARSENIC = As, BERYLLIUM = Be, BARIUM = Ba, CARBON = C, CHROMIUM = Cr, COBALT = Co, COPPER = Cu, CALCIUM = Ca, FLUORINE = F, IRON = Fe, LEAD = Pb, LITHIUM = Li, SODIUM = Na, MAGNESIUM =Mg, MANGANESE = Mn, MOLYBDENUM = Mo, MERCURY = Hg, NICKEL = Ni, STRONTIUM = Sr, SILVER = Ag, TIN = Sn, TITANIUM = Ti, TUNGSTEN = W, URANIUM = U, VANADIUM = V, ZIRCONIUM = Zr, ZINC = Zn
ALUMINUM = Al, ANTIMONY = Sb, ARSENIC = As, BERYLLIUM = Be, BARIUM = Ba, CARBON = C, CHROMIUM = Cr, COBALT = Co, COPPER = Cu, CALCIUM = Ca, FLUORINE = F, IRON = Fe, LEAD = Pb, LITHIUM = Li, SODIUM = Na, MAGNESIUM =Mg, MANGANESE = Mn, MOLYBDENUM = Mo, MERCURY = Hg, NICKEL = Ni, STRONTIUM = Sr, SILVER = Ag, TIN = Sn, TITANIUM = Ti, TUNGSTEN = W, URANIUM = U, VANADIUM = V, ZIRCONIUM = Zr, ZINC = Zn
ALUMINUM = Al, ANTIMONY = Sb, ARSENIC = As, BERYLLIUM = Be, BARIUM = Ba, CARBON = C, CHROMIUM = Cr, COBALT = Co, COPPER = Cu, CALCIUM = Ca, FLUORINE = F, IRON = Fe, LEAD = Pb, LITHIUM = Li, SODIUM = Na, MAGNESIUM =Mg, MANGANESE = Mn, MOLYBDENUM = Mo, MERCURY = Hg, NICKEL = Ni, STRONTIUM = Sr, SILVER = Ag, TIN = Sn, TITANIUM = Ti, TUNGSTEN = W, URANIUM = U, VANADIUM = V, ZIRCONIUM = Zr, ZINC = Zn CENTERING COLOR ELEMENTS GO TO DARK MINERAL ELEMENTS IN CRYSTAL, COLOR CHART INTERNAL ELEMENT COLORING, IDIOCHROMATIC (+) INTERNAL EXTERNAL ELEMENT COLORING, ALLOCHROMATIC (-) FOREIGN STANDARD PIGMENT COLORS (BOLD) ALUMINUM = AL ANTIMONY = Sb ARSENIC = As BERYLLIUM = Be BARIUM = Ba CARBON = C CHROMIUM = Cr COBALT = Co COPPER = Cu CALCIUM = Ca FLUORINE = F IRON = Fe LEAD = Pb LITHIUM = Li SODIUM = Na MAGNESIUM =Mg MANGANESE = Mn MOLYBDENUM = Mo MERCURY = Hg STRONTIUM =Sr TITANIUM = Ti TUNGSTEN = W URANIUM = U VANADIUM = V ZIRCONIUM = Zr ZINC = Zn
THE REAL COLOR WHEEL
Three primary colors for pigments, three primaries for light, in each case the primary colors combine and subdivide into the whole range that is shared by both systems. Three basic colors, divide to six, twelve, twenty four, thirty six, sixty or three-hundred-sixty part color wheels. Start with yellow at the top of the color wheel, and "Read Red Right", the three R's of the color wheel. We'll play the color wheel like a typewriter. There are only twelve colors to remember, forward backward and across the centering middle to the opposite side. Name a color and have an image of that color in your mind. Yellow is represented by "Y", Red is "YM", Magenta is "M", Ultramarine Blue is "MC", Cyan is "C", and "CY" is Green. A pure unadulterated yellow would be YYYY, half yellow and half magenta would be YYMM, or the color red, each rim color can have from one to four characters, depending on the wheel's total color size. Orange is YYYM, Scarlet Crimson is YMMM. The twelve color wheel is enough to paint any picture with pigments. Here, the sixth color away from any color is halfway around the color wheel, making it the opposite color or the complementary color. The opposite of Transparent Yellow, YYYY is MMCC, Transparent Ultramarine Blue. Opaque Yellow mixed with Ultramarine Blue opaque or translucent will make green. (See Painting on Location Section for full explanation) Split complementary colors are two colors next to each other called "analogous" opposite one color across the wheel. Here's an example, Green is opposite Magenta, the split complementary colors of Green are on each side of Magenta, Scarlet and Purple. THE LIGHT AND PIGMENT COLOR WHEEL'S ARE THE SAME COLORS PRIMARY, PIGMENT, THREE COLOR WHEEL Y = Yellow, M = Magenta, C = Cyan. Each color is 120 degrees, a primary triad division without opposition's. Any three pigment triads make a neutral dark, and three light primaries equal white light, Y+M+C = YMC. White in light, dark in pigment. PRIMARY, LIGHT, THREE COLOR WHEEL YM = Red, MC = Ult.Blue, CY = Green. The combined three triads equal White in light. (YM+MC+CY)=(YMC+YMC) = Bright White, White gets brighter, black is black. In light there are no degrees of black, only degrees of added light to black. SIX COLOR WHEEL, COMBINES THE LIGHT AND PIGMENTS TRIADS Each color has 60 degrees. Opposites are separated by two colors, #1=Y and #4=MC are complementary opposite colors. The formula, Y+MC = YMC, equals Neutral White in light, or Neutral Dark in pigment. COLOR SYMBOLS FOR A SIX COLOR WHEEL #1, Y = Yellow, #2, YM = Red, #3, M = Magenta, #4, MC = Ultramarine, #5, C = Cyan, #6, CY = Green, EACH COLOR HAS 30 DEGREES. OPPOSITES ARE SIX COLORS APART, #1 AND #7 ARE OPPOSITES IN LIGHT OR PIGMENT. EXAMPLE, Complementary colors added together. YY+MMCC = YY+MM+CC or YMC+YMC, two neutrals, no color. YYM+MCC = YY+MM+CC or YMC+YMC, two neutrals, no color, COLOR SYMBOLS FOR A TWELVE COLOR WHEEL, YY=Yellow, YYM=Orange, YYMM=Red, YMM=Scarlet-Crimson, MM=Magenta,
MMC=Purple, MMCC=Ultramarine, MCC=Azure, CC=Cyan, CCY=Turquoise, CCYY=Green,
CYY=Yellow-green. Each color is 15 degrees on the 24 RCW, Opposites are 12 colors apart, #1
and #13 are opposites. Combine any two opposition colors to get a neutral, white
in light, neutral dark in pigment.
COLOR SYMBOLS FOR A TWENTY FOUR COLOR WHEEL. Yellow plus Ultramarine Blue (4M4C) equals, (4Y)+(4M+4C) or 4YMC. A six colorwheel opposition would look like this, Y+MC = YMC. YMC, the primary colors in pigment combine into Neutral White in light or Neutral Dark in pigment. Pigment has 100% intensity in it's pure state, tube form. Mixing two pigments decreases the new colors intensity. Pigments are subtractive. In light, adding color to color makes it lighter and brighter. Light is additive. Twice as much added color will make a light twice as intense. In the combination, Red, Ult.Blue and Green (light's primary colors), (4Y+4M)+(4M+4C)+(4C+4Y) equals (8Y+8M+8C) or 8YMC. This combination is twice as bright as Yellow, Magenta and Cyan, (4Y)+(4M)+(4C) or (4YMC). 100% Red Light plus 100% Green Light equals 100% Yellow light, the Yellow light is twice as bright. Combining a less brightly lit colored light, 50% of each color Red and
Green will equal brown light. The most intensive Yellow light is made from the
less intensive 100% Cadmium Red Light and 100% Green light. Light adds to
intensity, so it's additive. (4Y+4M)+(4C+4Y) equals (4Y+4M+4C)+(4Y) or (4YMC)+(4Y), that's four
Neutrals, plus four Yellows, or Yellow Light.
CONVERSION OF LIGHT TO PIGMENT New Page, GO TO LIGHT TO PIGMENT CONVERSION, click here.
In the Painting on Location section. There you will find an interactive color selection.
100%, Green Light plus 100% Red Light, = 100% Yellow Light, twice as bright as the Green and Red Lights by themselves. Light is additive. 50% Green Light plus 50% Blue Light = 50% Cyan, a shade of Thalo Blue. This shade color in the light color wheel has the same green tainted dark that yellow has when it changes to black by subtracting light. Pigments don't subtract light so the RGB and YMC color wheels as they are, won't work for pigments.
10% Red Light plus 10% Green Light make a dark Brown/Green Light as that extension of Yellow Light. The "Real Color Wheel for Pigments" shifts this extension for yellow from the dark green/side to the dark brown/side by using the dark of red as the dark of yellow. Just as light does in physical crystals of the iron element, and others. Now you can use yellows opposite color in either light or pigment color
wheel and get a neutral dark mixture. Your yellows will not only turn nice
greens but you'll have the all the browns you need and were meant to have.
PIGMENT, DIVISION 1, Yellow to Magenta scale in 20 Sections. LIGHT, DIVISION 1, Green Light to Red Light scale in 20 sections. PIGMENT COLOR PERCENTAGES, DIVISION 1, Yellow to Magenta. Sections: #1=100Y, 2=100Y10M, 3=100Y20M, 4=100Y30M, 5=100Y40M, 6=100Y50M, 7=100Y60M, S=100Y70M, 9=100Y80M, 10=100Y90M, 11 =100Y100M, 12=90Y100M, 13=80Y100M, 14=70Y100M, 15=60Y100M 16=50Y100M, 17=40Y100M,, 18=30Y100M, 19=20Y100M, 20=10Y100M DIVISION 2, Magenta to Cyan Sections: 21=100M, 22=100M10C, 23=100M20C, 24=100M30C, 25=100M40C 25=100M50C, 27=100M60C, 28=100M70C, 29=100M80C, 30=100M90C 31=100M100C, 32=90M100C, 33=80M100C, 31=70M100C, 35=60M100C, 36=50M100C, 37=40C100C, 38=30M100C, 39=201~4100C, 40=10M100C, DIVISION 3, Cyan to Yellow Sections: 41=100C, 42=100C10Y, 43=100C20Y, 44=100C30Y, 45=100C40Y 46=100C50Y, 47=100C60Y, 48=100C70Y, 49=100C80Y, 50=100C90Y, 51=100C100Y, 52=90C100Y, 53=80C100Y, 54=70C100Y, 55=60C100Y, 56=50C100Y, 57=40C100Y, 58=30C100Y, 59=20C100Y, 60=10C100Y. These are the 60 rim colors of this Real color wheel. The Real color Wheel has 3-6-12-24-36 or 360 colors. A Neutral Dark is made by mixing complementary pigments anywhere along the Real Color Wheel. For the pigment artist the pigment Opaque Yellow graduates into the color of the pigment Burnt Umber Brown before turning Neutral Dark (with an equal mix of Ult.blue). Red graduates to this Brown naturally in light and pigment, while Yellow graduates to Green then Black in light.. Neutral Dark colors can be made by mixing the complementary colors in light or pigment. The RCW makes it possible to join the light and pigment color wheels. They are the same color wheel if you make the adjustment to Neutral Dark in the yellow scale of the light color wheel and don't use the pigment Black in the pigment color wheel. Now that's a RCW for all artists! An opaque pigment is a dense solid, like a rock. A transparent pigment you can see through, like glass, water, dye or stain. A "Tyndall" beam of light will pass through a transparent solution unseen. A translucent is colloidal, particles of solid so small there continually suspended but not dissolved. Milk is a colloidal, solid fat dispersed in clear water, add more water and it becomes more translucent. It can always be more translucent but never transparent, the beam of light will always show the particals. Vaper can be a colloidal transparent. This is an illustration of what is being done to the natural line of color progression in the RCW. Yellow now stays on the warm red side while becoming dark. A line from corner to corner diagonally on this chart would be the RGB line of progression, the Yellow turning green as the RGB neutral is. With this curve in place, all the Yellow colors getting dark lean to the warm side allowing the Naples, Ochers and Umbers to show up under Yellow. This does not happen in the RGB color wheel and that is one of the reasons why the artists color wheel and the light color wheel would never match. A similar curve exists for the Cyan to Cyan Black verses Cyan to Cyan Blue Black, I got rid of the Green Black in Cyan and changed it to a Blue Black so it matched the sky and the pigment transparent Cyan (Thalo Blue). 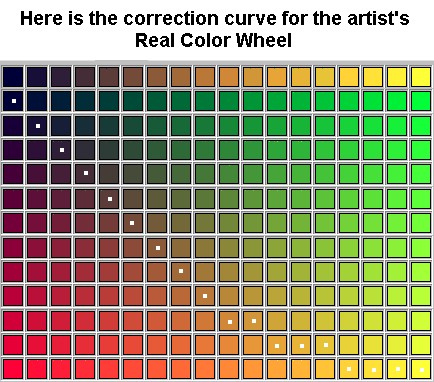
Here are some permanent chemical pigments used in oil and acrylic paints
today. PY3 stable di-arylide = Yellow Lemon opaque on barium sulfate, Gamboge PY83 stable di-arylide = Yellow Deep, Madder Lake, Alizarin Crimson, Italian Brown Pink Lake. PY153 dioxine nickel complex = Indian Yellow Golden/s or Brown/s with PY142 translucent iron oxide, it also makes Gamboge, Indian Redgold, Sap Green, Indian Yellow Green PY153 dioxine nickel complex with PO69 isiondolin = makes Indian Yellow, Org/side PY153 dioxine nickel complex with PR 260 isoindolin = Indian Yellow Golden, Vermilion to Red Scarlet dual-toned PY129 methin copper complex = Golden Green, Indian Yellow Green with PY153 PR101 synthetic iron oxide = Translucent Red-Yellow to Brown PY42 synthetic iron oxide = Translucent Yellow to Brown Transparent colors are precipitated on alumina, the oxide of aluminum present in clay. Another base for these colors could be cyclohexanone, wax, or an acrylic polymer emulsion. PY150 azo complex = Indian Yellow Brown side. BEST PY153 dioxine nickel complex = Indian Yellow Orange side. BEST PY153 dioxine nickel complex + PY42 translucent yellow oxide = Indian Yellow Brn/s by Old Holland . PY153 dioxine nickel complex + PR260 isoindolin = Indian Yellow Golden. PY150 dioxine azo complex + PY42 synthetic iron oxide = Indian Yellow Brown. PY153 dioxine nickel complex + PY3 stable di-arylide = Gamboge. PY83 stable di-arylide + PR101 synthetic iron oxide = Italian Brown Pink Lake.
PY150 nickel azo = Indian Yellow Brown. BEST PY153 dioxine nickel complex = Indian Yellow Golden or Orange side. BEST PY153 dioxine nickel complex + PR 260 isoindolin = Indian Yellow Golden. PY95 nickel azo condinsation + PR 101 iron oxide = Indian Yellow Golden. PY153 dioxine nickel complex + PY3 stable di-arylide = Gamboge PR 170:F5rk naphthol carbamide = Scarlet Pink PV19 quinacridone = Rose PR122 quinacridone = Magenta PV23:1r carbazole dioxazine = Purple PV23 dioxine nickel complex = Permanent Violet Bluish transparent secondary blue, tints to Ult. Blue. PB60 anthraquinone = Blue Deep to Turquoise PB15 copper phthalocyanine = Cyan (Thalo Blue) to Green Y/S PB7 Chlorinated copper phthalocyanine = Turquoise to Green PY83 stable di-arylide + PG7 chlorinated copper phthalocyanine > = Sap Green Y/S PY83 stable di-arylide HR + PG7 chlorinated copper phthalocyanine + PO43 perinone orange = Sap Green O/S PY129 methin copper complex = Green Gold PY129 azomethine = Genuine Green Gold PY35 cadmium Zinc sulfide = Cadmium Yellow Lemon PT37 cadmium sulfide = Cadmium Yellow Light, Medium PO20 cadmium sulfo-selenide = Cadmium Orange PR108 cadmium seleno sulfide = Cadmium Red Light, Medium PR101 synthetic red iron oxide = Red Oxide PY42 synthetic yellow iron oxide = Yellow Oxide PBr7 natural iron oxide, raw and calcined = Siena and Umber PB29 silica, aluminium, sulphur complex = Ultramarine Blue PB28 oxides of cobalt and aluminum = Cobalt Blue PG17 anhydrous chromium senquioxide = Chromium oxide Green
Interactive Real Color Wheel Matching Tube Pigment Colors in RGB and YMC.Each colors number relates to it's position on the 36 color Real Color Wheel. These 36 RCW colors get dark just as they do in crystals. Opposite complement pigments mix dark shadow colors just as nature makes shadows on sunlit items. The top color Yellow is the color numbered (1a.) The number (1b) is a darker version of (1a) on the way through the dark middle to it's opposite color, (19a) Ultramarine Blue. Just as the Titanium Crystal, Rutile
Test your monitor, the top button is the deepest Black. The lower radio button is White. Go back to White.
Top-tone is adding White to the color. Under-tone is adding clear. Mass-tone is thick out of the tube. 01a. Bellini Lemon Yellow, Lead Chromate, a light cool Yellow. #"FAFF28", RGB, R=255 G=255 B=000, CMYK= 80Y 20M 01a. YYYY, Grumbacher Cadmium Barium Yellow Pale, a middle Yellow. #"FFFF0E", R=255 G=255 B=014 01a. YYYY, Mussini Cadmium Yellow Lemon, the middle Yellow. #"FFFF00", R=255 G=255 B=000, CMYK=Y 01b. Mussini Zinc Yellow, the coolest Yellow. An extra color. #"F1F10A", RGB, R=241 G=241 B=010 01b. Mussini Cadmium Yellow Pale, a slightly cool Yellow. #"FFFA43", R=255 G255 B=040 01c. Old Holland Gamboge Lake Extra, a Yellow-Orange dual-tone changing to a Cadmium Yellow Under-tone. #"FF9F00", R255, G255 B000
1c. Old Holland Indian Yellow-Orange Lake Extra, a dual-toned Orange to Yellow transparent color. #"FF5700", R=255, G=087, B=000 The pale undertone is a strong Yellow-Pale while the top mass-tone hue is Orange and Vermilion mixed 1:1. This color can replace the famous "golden transparent", one of the two color aspects of Natural Indian Yellow. Here is
the dual-toned Under-tone color of Old Holland Indian Yellow-Orange Lake
Extra. #"FFF000", R=255, G=240, B=000
1e. Rembrandt or Mussini Asphaltum, the ancient color Bitumen, a transparent dark brown/yellow side, Rembrandt used this favorite glaze to transcend dark (Doerner). The Smithsonian says he did not use this color? #"331D00", R=051, G=029, B=000. New in Liqutex, Van Dyke Red Hue, mixes neutral with Ultramarine Blue. Way to go Liqutex! A transparent
Under-tone of Rembrandt or Mussini Asphaltum and the new Benzimidazolone
PBr:25 is clickable below
1e. Mussini Burnt Umber, a calcined translucent warm Red-Brown. Note: Cadmium Yellow also darkens to this color in the "Real Color Wheel" using this Brown to form all warm Yellows. #"331500", R=051, G=021, B=000, CMYK= Y M C 02a. Mussini Naples Yellow Light, an opaque Nickel and Titanium replacement for Antimony Yellow. This is the first of eleven pre-made with added white colors, they must be added to a complete painting color palette. This was the traditional (1875) base flesh tone when made with Antimony Lead, later and earlier lead-tin Yellow was used. #"FFEDA8", R=255 G=237 B=168, CMYK= 60Y 20M 03a. Mussini
Naples Yellow Deep, (True Naples is either Green-side, Yellow-side or Red-side,
higher firing temperatures make warmer, lighter colors. #"D9A878",
R=217 G=169 B=120, CMYK= 60Y 40M
2b. Schmincke Mussini Cadmium Yellow Middle is more Yellow than Grumbacher Cadmium Yellow Medium. #"FFED09", R=255 G=337 B=009, CMYK= Y 20M 05a. Grumbacher
Cadmium Yellow Medium. #"FFE709" R=255 G=231 B=009
02c. Mussini Yellow Raw Ocher, opaque tan. #"9B6A26", R=155 G=106 B=038, CMYK= 80Y 20C 40M 02d. Mussini Translucent Yellow Oxide, dual-toned red-tan, add white for a yellow-tan. It has the same mass-tone matching color as Mussini Indian Yellow Brown Lake Extra. #"986011", R=152 G=096 B=017 A top-tone
of Mussini Translucent Yellow Oxide is clickable below
4a. YYYM, Mussini Cadmium Orange, opaque. #"FF8200", R=255, G=130, B=000, CMYK= Y 60M 4b. Mussini Burnt Sienna, transparent Red-Brown. Orange changing to dark brown, goes through Burnt Sienna. Above click is the mass-tone from the tube. #"4D0000", R=077, G=000, B=000, CMYK= Y 60C M To See an Under-tone (the color with clear medium) of Mussini Burnt Sienna, click below. Below is
a high Top-tone tint of Mussini Burnt Sienna.
6a. Rembrandt Chinese Vermilion Extra, translucent bright Red Light. #"FF3200" R=255 G=050 B=000, CMYK= Y 80M 07a. YYMM, Cadmium Red Medium. #"FF0000" R=255, G=000, B=000, CMYK= Y M 7b. Blocks Venetian Red, warm opaque. Cadmium Red going to neutral dark black. #"A60000", RGB, R=153, G=000, B=000, CMYK= Y 40C M 10a, YMMM, Rembrandt Rose, transparent Scarlet-Crimson. #"F1004C", R=241, G=000, B=076, CYMK= 90M An Under-tone of this tube color is here below. Here below
is a light Top-tone of Rembrandt Rose, the perfect Pink, notice it is getting
cooler. #"FF4186", RGB, R=255, G=065, B=134
12a. MMMM, Danial Smith Quinacridone Magenta, a warm dual-tone Magenta with a cooler mass tone similar to Cobalt Violet and a warmer top-tone, it mixes to a perfect neutral dark with Thalo Green. #"BD0053" R=189 G=000 B=083, CMYK= M. All complementary colors make perfect neutral grays and blacks, that can be adjusted warm or cool. 13a. MMMM, Bocour, New York, Cobalt Violet Phosphate, transparent dual-toned, dark mass-tone to a cool Top-tone pink when mixed with White and a warm Under-tone pink when mixed with medium. This color is perfect for making Ultramarine Blue using Thalo Cyan. #"BD0081" R=163, G=000, B=109, CMYK= 10C 90M Cool Top-tone
below
Cooler Top-tone with more White below,
Warm transparent Under-tone below
16a. MMMC, Liquitex Carbazole Dioxazine Purple, transparent. With the addition of White, this pigment completely changes into a cooler color Purple, it is dual-toned. #"300030" R=048, G=000, B=048, CMYK= 80C M A tint
Top-tone color of this pigment is this radio button below.
16a. Grumbacher Dioxazine Purple, the same mass color as #13, this tube color looks like this. With the addition of White this pigment fills the warm slot. The tinted color of this pigment is this radio button.
18a. Ultramarine Violet, translucent, from the tube. #"260035", R=038 G=000 B=053, CYMK= 80C M A Top-tone
tint of this color is:
19a. MMCC, Blocks French Ultramarine Blue, translucent. #"150036", R=021, G=000, B=036, YMCK= C 80M A Top-tone
tint of this color is:
19b. Mussini Ultramarine Light, translucent. #"150064", R=021, G=000, B=100 A Top-tone
tint of this color is:
22a. Mussini Cobalt Blue Light, opaque. Nobody makes a true Cobalt Blue anymore, the artificial colors are in this Opaque Azure range, there are no Transparent Azures available. #"00255C", R=000, G=037, B=092, CMYK== C 80M A Top-tone
tint of this color is:
25a. CCCC, Grumbacher Thalo Blue, transparent, a perfect Cyan. This colors Mass-tone is Ultramarine Blue transparent, and the Top-tone tint color is RGB Cyan, just like the sky. The more white that is added, the warmer the color becomes. #"000040", R=000, G=000, B=064, CYMK= C. Paint the sky with Thalo Blue and Dioxine Purple to make all the colors between and including the Ultramarine Blue color. In crystal Cyan darkens by adding magenta's dark. A middle Top-tone tint of this color: A very
high Top-tone tint of this color is, RGB, Cyan:
27a. CCCY, Rembrandt Blue Green, a transparent Turquoise color. #"003020", R=000, G=048 B=048, CYMK= 20Y C A lighter
tint of this color is:
29a. Mussini Opaque Green Light, a mix of Titanium and Phthalo Green, cool opaque, too handy to be in the auxiliary color wheel. #"007559" R=000, G=117, B=089, CYMK= 60Y 80C 31a. CCYY, Mussini Phthalo Green is Blue/side, transparent. #"003010" R=000. G=048, B=016, CYMK= Y C A tint
of this color is: RGB, 000, 225, 233,
32a. Grumbacher Thalo Green Yellow/Side, transparent. Has a warmer mass-tone than Top-tone . #"003000" R=000, G=048, B=000 A Top-tone
tint of this color is: RGB, R=000,G=255, B=176
A tinted
Top-tone by adding white of this color is: RGB, R=000,G=255, B=147
34a, Rembrandt Chromium Green Oxide, warm opaque. #"009B7A", R=000, G=080, B=000, CMYK== Y 80C 40M 34a. Grumbacher Green Earth, warm translucent. #"3E5600", R=062, G=086, B=000 35a. CYYY, Old Holland Yellow-Green Organic Opaque has a cooler mass-tone than Under-tone. #"79C700" R=121, G=199, B=000, CYMK= 20C Y, Here below is the warmer Under-tone mixed with a clear medium. 35b. Winsor and Newton Sap Green, #"233A00, R=035, G=058, B=000, transparent dual-tone, from a mass-tone cooler dark to a warmer tint mixing Yellow-Green and White. Caution; Most brands include black in this pigment and don't give it a permanent rating. Be safe and don't use it or use a synthetic. A tint
with added white of this color is: RGB, R=152, G=197, B=000
36a. CYYY, Brillant Yellow-Green, transparent in acrylic, opaque in oil. #"DBFF76" R=121, G=255, B=028, CYMK= 10C Y 36b. Mussini Genuine Golden Green, #"606300", R=096, G=099, B=000, CYMK= Y 40C 40M A Top-tone
tint by adding white to this dual-toned color is: RGB, R=246, G=255, B=000
36c. Mussini Raw Umber, a translucent cool Brown/green side. (Yellow-Yellow-Yellow-Green darkens to this color in my light to pigment color wheel). #"332500", R051, G=037, B=000, CMYK= Y 80C 80M These colors
make painting very easy indeed. All dots are
links to each pigments discripton. Clickable Artists Real Color Wheel matching pigments
PIGMENT COLOR LINKS IN RGB RCW#1.00.1, Bellini Lemon Yellow Lead Chromate, Oil, Opaque, PY-34
RCW#1.00.1, Light Chrome Yellow Light, Lead Chromate, PY-34
RCW#1.0.1, Mussini Zinc Yellow, Opaque
RCW#1.0.2, 1.0.2 YYYY, Hansa Yellow, Monoazo Yellow, PY-74, Translucent
RCW#1.0.4, Light, Yellow Light Hansa, Arylamide, PY3, Translucent
RCW#1.0.5, Nickel Titanate Yellow PY53
RCW#1.0.6, YYYY, Grumbacher Cad. Barium Yellow Pale, Opaque
RCW#1.2, New Gamboge, Anthrapyrimidine PY108, O.H. Gamboge Lake Extra, organic, Translucent.
RCW#1.3.5, Old Holland Indian Yellow-Orange Lake Extra, Dioxine Nickel Complex, Isindolin, dual-toned Transparent, tint.
RCW#1.4, Warm Gold ocher - Azo Yellow, Monoazo Yellow, PY-151, Translucent RCW#1.6.1, Indian Yellow Gr/s, Nickel Chelated Azo PG10F, transparent. Very little green side.
RCW#1.8, Old Holland Indian Yellow-Brown Lake Extra, Dioxine Nickel Complex + Synthetic Iron Oxide, dual-toned transparent, tint.
RCW#1.9 Rembrandt or Mussini Asphaltum, Transparent PBr7
RCW#1.10.5, Burnt Umber Brown, Natural Earth, Hydrated Iron Oxide, PBr7, Translucent
RCW#2.00.5 Mussini Naples Yellow Light, Titanium Dioxide, Rutile-Nickel-Tin-Titanium, Chromium-Antimony-Titanium Yellow PW6, PY53, PBr24, Opaque
RCW#2.0, Schmincke Mussini Chrome Yellow Orange Lead Chromate, PY34, Opaque
RCW#2.3, Mussini Translucent Yellow Oxide, Translucent
RCW#2.4, Mussini Yellow Raw Ochre, Natural Hydrated Iron Oxide, PY43, Mussini Translucent Yellow Oxide, Translucent
RCW#3.00, Mussini, Naples Yellow Deep, Lead Antimonate, PY41, Opaque
RCW#3.0, Cadmium Yellow Deep, Opaque
RCW3.3, Raw Siena, Natural Iron Oxide, PBr7, Opaque
RCW#3.4.5, Italian Deep yellow Ocher R/s, Opaque
RCW#3.6, Quinacridone Deep Gold PO48, Transparent
RCW#3.7, Brown Oxide PR101, Transparent
RCW#3.10, Mussini, Burnt Umber, Natural Earth, Hydrated Iron Oxide, PBr7, Translucent
RCW#4.0.1, Isoindolinone Yellow R, PY110 transparent, mass-tone
RCW#4.0.4, Benzimidazolone Orange, PO62, Transparent
RCW#4.0.5, YYYM, Mussini Cadmium Orange, Cadmium Sulfo-Selenide, PO20, Opaque
RCW#4.6, Burnt Sienna PBr7, Translucent
RCW#4.10, Burnt Umber Brown, Natural Earth, Hydrated Iron Oxide, PBr7, Translucent
RCW#5.7, Italian burnt Sienna PBr7, Translucent
RCW#5.10, Burnt Umber Brown, Natural Earth, Hydrated Iron Oxide, PBr7, Translucent
RCW#6.0.5 Vermilion Extra, Isoindolindon, PR260, Translucent
RCW#6.10, Burnt Umber Brown, Natural Earth, Hydrated Iron Oxide, PBr7, Translucent
RCW#7.0.5, YYMM, Cadmium Red Medium or Light, Opaque
RCW#7.0.6, Thioindagoid Red PR88, opaque
RCW#7.5, Warm Red Oxide PR:10, opaque
RCW#7.6, Red Oxide - Blockx Venetian Red Warm, Synthetic Iron Oxide, PR101, Opaque
RCW#7.9, Bemizimidazolone Brown PBr:25, opaque
RCW#7.10.5, Burnt Umber Brown, Natural Earth, Hydrated Iron Oxide, PBr7, Translucent
RCW#8.0.5, Irgazine Red PR254, Opaque
RCW#8.10, Burnt Umber Brown, Natural Earth, Hydrated Iron Oxide, PBr7, Translucent
RCW#10.00, Light Portrait Pink, Naphthol Red AS-D + Titanium White + Diarylide Yellow, PR:122, PW:6, PY:83, Opaque
RCW#10.0.5, Naphthol Crimson, Naphthol AS, PR170, Translucent
RCW#12.0, YMMM, Rembrandt Rose, Permanent Rose, Translucent Scarlet-Crimson, Quinacridone Rose Bata, PV:19, Transparent
4RCW#13.00, Light Magenta, Quinacridone Violet, Titanium Dioxide, PR122 Y, PW6, Opaque
RCW#13.0, MMMM, Quinacridone Magenta Y, PR122, Transparent
RCW#14.00.5, Cobalt Violet, PV49, Opaque Cool Magenta
RCW#10.5.22, Cobalt Violet, Cobalt Phosphate, PV14, Transparent Cool Magenta, dual-toned
RCW#15.0, Manganese Violet PV23 R/s, Top-tone, Transparent
RCW#15.6, Manganese Violet PV23 R/s, Mass-tone, Transparent
RCW#16.0, MMMC, Dioxazine Purple, Carbazole, Warm, PV23, Top-tone, Transparent
RCW#16.6, MMMC, Dioxazine Purple, Carbazole, Warm, PV23, Mass-tone, Transparent
RCW#18.0, MMCC, Blockx,Ultramarine Violet, Alumosilicate of Sodium, PV15, Top-tone, Transparent
RCW#18.6.5, MMCC, Blockz, Ultramarine Violet, Alumosilicate of Sodium, PV15, Mass-tone, Transparent
RCW#19.00, Light Blue Violet Acrylic, Ultramarine Blue + Titanium Dioxide, PB29, PW6, Opaque
RCW#19.0, French Ultramarine Blue PB29, Translucent
RCW#19.2.5, Ultramarine Blue Light PB:29, Opaque
RCW#19.3.5, Ultramarine Blue Deep PB29, Complex Silicate of Sodium and Aluminum with Sulfur, Translucent
RCW#22.0, Cobalt Blue PB28, Oxides of Cobalt + Aluminum, Opaque
RCW#25.00.5, King's Blue Deep, Tint, Zinc Oxide + Titanium Dioxide Rutile + Synthetic Ultramarine PB29, PW4, PW6, PB29, Opaque
RCW#25.01.5, Cerulean Blue PB-36, Oxides of Cobalt + Chromium Opaque PB:15.3, Opaque
RCW#25.0, CCCC, Thalo Blue - Phthalo Blue, Cyan PB:15.3, Pathalocyanine, Top-tone, transparent
RCW#25.0.3, CCCC, Old Holland, Manganese Blue, Barium Manganate PB:33, Cyan Top & Mass-tone, Transparent
RCW#27.0.5, CCCY, Rembrandt Blue Green, Turquoise PB15.3 + PG36, Pathalocyanine, Top-tone, Transparent
RCW#27.6, CCCY, Rembrandt Blue Green, Turquoise PB15.3 + PG36, Pathalocyanine, Mass-tone, Transparent
RCW#28.03, Bright Aqua Green, Phthalocyanine Green + Phthalocyanine Blue, Titanium Dioxide, PG7, PB15, PW6, Opaque
RCW#29.03, Mussini Opaque Green Light, Phthalocyanine Green, Monoazo Yellow, Cobalt-Titanium-Nickel-Zinc-Aluminum-Oxide, Opaque
RCW#31.03.5, CCYY Tint, Emerald Green, Brominated Copper Phthalocyanine, Titanium Dioxide, PG36, PW6, Opaque
RCW#31.0.1, CCYY, Mussini Phthalo Green, Y/S, Brominated Chlorinated Phthalocyanine, PG36, Transparent
RCW#31.0.5, CCYY, Mussini Phthalo Green, Phthalocyanine Green, PG7, Transparent
RCW#33.3, Mussini Permanent Green Light, PG-7 + PY-3, Phthalo Green + Monoazo Yellow, Opaque
RCW#33.6, Hooker's Green Permanent PG36 + PY3 + PO49, Transparent
RCW#34.4, Rembrandt Chrome Oxide Green, PG-17, Chrome Oxide, Opaque
RCW#35.0, YYYC, Yellow-green Organic, Opaque Hooker's Green, PY73, PG7, PR101, Arylamide Yellow GX + Phthalocyanine Green + Red Oxide, Opaque
RCW#35.6, Grumbacher Green Earth Natural, high silicone clay, PG:26, Translucent
RCW#35.10, Winsor and Newton Sap Green Syn. Transparent, Duel-tone
RCW#36.00.8, Priderit Yellow PY157, Yellow-green, Opaque
RCW#36.0.5, Brilliant Yellow Green, PG7, PW6, PY3, PY97, Chlorinated Copper Phthalocyanine, Titanium Dioxide, Arylide Yellow 10G, Translucent in Acrylic, Opaque in Oil
RCW#36.4.9, Old Holland Indian Yellow-Green Extra, Dioxine Nickel Complex, Methin Copper Complex, PY153, PY129, Transparent
RCW#36.6.9, IrgazineGreen PY129, Dual-tone, Translucent
RCW#36.8.5, Golden Acrylic Green Gold, Nickel Azo PY:150 + Thalo Gr.Y/s PG:36, Hansa Yellow PY:3, Dual-tone, Translucent
RCW#36.9.5, Mussini Golden Green Genuine, Azomethine Metalcomplex, PY129, Azomethine Copper Complex, Transparent
RCW#36.10.9, Raw Umber Brown, Natural Earth, Hydrated Iron Oxide, PBr7, PY42, Translucent
RCW#36.10.1, Green Umber, Translucent
RCW#35.10
.
Top-tone is adding White to the color.
COLOR CHAPTER#36 PAGE INDEX FOR CHAPTER #36 36-02, MEASURING COLOR, SPECTROSCOPE, 36-03, MEASURING COLOR, SPECTROSCOPE, 36-04, LIGHT TERM GLOSSARY, 36-05, LIGHT TERM GLOSSARY, 36-06, LIGHT TERM GLOSSARY, 36-07, PRISMS, 36-08, TOP VIEW, 36-09, TOP-SIDE, 36-10, BOTTOM VIEW, 36-11, RIGHT ANGLE, 36-12, INTERNAL POLARIZED LIGHT EDGES, 36-13, POLARIZED LIGHT EDGES, 36-14, POLARIZED LIGHT EDGES, EXTERNAL SPECTRUM, 36-15, LIGHT IN A SPHERE, 36-16, RAINBOWS, 36-17, RAINBOW POSITION, 36-18, SECOND REVERSED RAINBOW, MEASURING_LIGHT_WAVES YYYY=Yellow, YYYM=Orange, YYMM=Red, YMM=Scarlet/Crimson, MM=Magenta, MMC=Purple, MMCC=Ultramarine Blue, MCC=Azure, CC=Cyan, CCY=Turquoise, CCYY=Green, CYY=Yellow-green, The top and bottom of a spectral analysis will show Red, YYMM, on top and Ultramarine Blue, MMCC, on the bottom. Combine the two colors like this, (red YYMM+MMCC ultblu) =is the same as saying= (YMC+YMC+MM). YMC stands for one Yellow, one Magenta and one Cyan, they combine to equal one white light. Combine these two white lights and the one magenta light and you get a higher intensity magenta light. Ultramarine blue light and red light combined to make the higher intensity magenta light. We're exchanging chroma for intensity, there isn't enough chroma in lights secondary colors to make a primary color, but there's enough chroma in the primaries to make a secondary color. Thomas Young and von Heimholtz developed the wave theory of light in 1890. The visible spectrum is measured by a spectroscope in wavelengths, light travels 300,000 kilometers per second, each visible color has a different wavelength distance between peaks. Very small distances are measured in angstroms, one trillionth of a millimeter. Visible light ranges from 3,800 angstroms for Violet (Magenta, called Violet) to 7,700 angstroms for Red light. 4100 angstroms = Ultramarine Blue, 4500 = Cyan, 5000 = Green, 5700 = Yellow, 6000 = Orange, 7000 = Red, 7600 = Red. This system explains the progression of colors in the rainbow. Another measurement system measures in nanometers, red being 7x10 (-7)m or 760 nanometers, the longest waves. Red begins at 760 nm, it's strongest and most intense at 600nm and ends at 460nm. Green is most intense at 520nm, it reaches from 425nm to 675 nm. Blue ends at 380 nm, it's strongest at 430nm and starts at 540nm. This system measures wavelengths and produces colors in light, such as a TV. These light dots combine optically not physically, to produce a full range of colors. LIGHT TERMS GLOSSARY PULSES of emitted light are like packages shipping off energy, the next one won't leave until the proton supply is built up again. INCIDENT light rays are parallel from the sun. The angle of incidence is equal to the angle of reflection. NORMAL is adjacent to the surface or horizon line. Normal is also perpendicular to the earth. Normal is straight up. OBLIQUE is not normal. Newton said light had particles going in a straight line, I agree. He didn't notice the diffraction, the missing magenta color or have a name for cyan, but he did notice separated colors could be recombined back into white. DIFFRACTION is a light ray angling around a solid object, the ray is white, the shortest wave to angle out of white is sometimes green, the "green flash" seen on the ocean horizon at the moment of sunset. Yellow and orange angle a little but red, the longest wave, bends the most. Warm Magenta is outside the red on the rainbow, and is the last color seen before the dark of night. This is color diffracting, in real time, focusing on a spot inside the shadow's void behind the earth, the center of diffraction. Diffraction enhances a painting rule, "It's darkest next to the light and lightest next to the dark". Diffraction also is the only way to see the light color magenta in it's pure state. Diffraction is what happens to a ray at it's light stopping edge, an object. The longest wavelength color can make the greatest diffracted angle. Magenta waves are still angling out of the straight white light already passed the stopping edge of the earth. They are spending more time in the reflecting atmosphere, on there way to a principal focus point on the center line's axis, deep in the heart of the earths shadow. The longest red rays refract with the greatest angle out of a prism, than yellow and green rays, magenta is the color in the center of an interferometer's white test for principal focus of diffracting dispersed light and making INTERFERANCE colored light bands. Yellow is the combination of red and green light. The interferometer combines and separates magenta and green light with a clear band of no light, yellow and cyan are in the green light, red and blue are in the magenta light. The whole spectrum is represented by four bands, magenta, white, green, and clear. Magenta is in the center of a diffraction record, made on an interferometer. First magenta, white, green and than a dark space, magenta, white, green, dark, etc. White light from the sun comes in transparent vibrating waves or rays, they're parallel, pulsed and diffracting, not bending, but emitting from the wave peaks. Red peaks out a little farther and reaches greater angles. A LASER'S beam is a continuous monofrequency, monochromatic, and parallel light. It's a light that will only go in a straight line, it does not diffract and lose color. A laser produces this beam, and turns it on and off, making "bits" of light, carrying coded information. This light is internally reflecting, never leaving a straight glass fiber cable. Total INTERNAL REFLECTION will keep an obliquely angled ray from leaving the reflecting skin of a substance. Light under water aimed with a narrower than 45 degree angle of reflection, will not leave the surface of the water but stay internally reflecting. A beam of light at 46 degrees will break the water surface at 1 degree, 44 degrees will stay internal. The REFRACTION ANGLE doesn't change inside the transparent substance, but at the skin of the substance. CENTER OF CURVATURE, is the focusing point of a sphere or concave mirror. Lens's are converging, diverging or flat. Converging lens's are thicker in the center, a sphere focuses in it's center, as a droplet of water or a crystal ball. DISPERSION of wavelengths and rays happen when the spectrum is divided in two by a substance. Red and yellow on one side, cyan and ultramarine blue on the other. INTERNAL PRISM SPECTRUMS happen in the transparent phases of the prism, looking through a prisms two planes angled at 45 degrees to each other. You can't see through two 90 degree planes, because the second plane is a perfect mirror. EXTERNAL SPECTRUMS happen with sunlight off a plane in the 46 degree or over reflecting phase, or through two transparent planes at a 45 degree angle. ??RIDDLE?? The hypotenuse is not a right angle but can be seen from a right angle. Right? Right! 90 degree is right angle. PRISMS Take a right triangle prism made of glass, plastic or diamond, and it will have one "right angle", or 90 degree angle, and two 45 degree angles, or, one hypotenuse and two inclined planes at 45 degree each. The left and right sides of the top view. A right angled prism's ratio is 3:4:3, two sides and the hypotenuse. The side to height ratio is, height three, side four. The hypotenuse divided by two equals the prisms height. The top view is over the hypotenuse, the bottom view is through it. All three plains are transparent in the top view over the hypotenuse until the line of demarcation at forty-five degrees from top, exactly 90 degrees perpendicular to the inclined plane, changes the hypotenuse into a mirror. Spectrum edge lights are seen through transparent phases. Red is on the compressed edge of transparency, blue is on the expanded side. Polarized edge spectrums happen on vertical and horizontal edges, not both at the same time, they are opposites. Refracting angles happen inside the prism each time light passes through the surface skin. PRISM, TOP VIEW Draw a line on paper and center the prism's bottom hypotenuse and top 90 degree angle line on it looking straight down on the prism perpendicular to the hypotenuse, notice there are two lines, not one, 30 degrees apart. You are looking down into the two 45 degree inclines, from a 90 degree perpendicular to the hypotenuse. The left side's image size has increased 15 degrees past center into the right, and vice versa, they overlap. The full vertical view through the side plane has increased from 100 degrees to 120 degrees, the total area is compressed and includes the overlap. Divide the hypotenuse area into three equal spaces and the prism's top view will increase it to four, by refraction. There is no distortion from a top 90 degree, entry. From the top you see through two transparent 45 degree planes. The hypotenuse plane is also transparent. This entry and exit is transparent and will be for forty-four and a half more degrees, compression left, by mirror entry right. This top position is good for seeing internal spectrums and producing two external spectrums at the same time, each projected 7 1/2 degrees off the hypotenuse, to the left and right. Perfect symmetry of double spectrums occurs from perfect angles of incidence, from the top straight down (+-) 7 1/2 degree, from the bottom, parallel to the sides (+) 7 1/2 degree. Top-vertical view, there's only this one degree of no distortion, compression starts immediately, there's 7 1/2 degrees of condensed spectrum before the mirrored line of demarcation removes it. The compression ratio equals twice the distance at half the compression. TOP-SIDE VIEW Now rotate the prism so a short side is vertical and touching the drawn line, you're looking down on the long hypotenuse side at a 45 degree incline. Continue the line around the prism and add two more parallel lines inside, dividing the outline into three equal spaces. The three spaces appear to have become four, by adding +15 degrees of refraction. This forth space is the vertical short side being refracted, adding 33% into the condensed view. The three thirds are condensed to four fourths to fit. The added fourth side is not transparent, it's a 15 degree refracted image, refracted through the skin of the 45 degree inclined hypotenuse, and it's an internal mirror. It will stay a mirror for the next 44 degrees of rotation. The top-side, right angle view of the 45 degree incline, shows the line of demarcation. The lower transparent half stops with the spectrum on it's leading edge. This is the prism angle. Two mirror image's start on the next degree and move the transparent section out of view in 7 1/2 degrees, they share the plane progressively till the 0 degree. Top-side view, a 37 1/2 degree incident light on a 45 degree plane, that's 7 1/2 degrees short of perpendicular to the side plane, transparent compression has made a window for a spectrum to project through, it emits through the bottom hypotenuse at a 7 1/2 degree angle, compressed and shining. BOTTOM VIEW Looking through a prism's hypotenuse, bottom first. Both sides are mirrors for 7 1/2 degrees of tilt off dead center. Upon tilting one side or the other will start a line of transparency, the mirror will be like a window shade going up with transparent compression under it, the spectrum is on that decompressing transparent line. The reflection portion of the plane doesn't compress, it forces compression on the transparent section from the line of demarcation onward. Compress the two opposing and trailing half spectrum edges of transparency by squeezing out the middle light with the line of demarcation's mirror edge, and the white light will become the green center. By compression, the two half prism lit edges form a complete spectrum. This compressed 1 degree span can emit a projected spectrum. Position; the prism is standing on it's end with the hypotenuse on the sun side. The sun's light enters from 90 degrees to 97 1/2 degrees, 7 1/2 degrees to the right of center, into the hypotenuse plane. The right underside plane stays a mirror at 7 1/2 degrees, the left underside becomes transparent. Two 7 1/2 degree spectrums are leaving the left side's plane simultaneously, both only 7 1/2 degrees off the plane's 45 degree angle to the hypotenuse and 180 degrees opposite each other. The left spectrum is reflected off the right mirror. The right spectrum is direct, with the sun going through two transparent planes. So, the sun enters 7 1/2 degrees off perpendicular, and exits 135 degrees later with a 7 1/2 degree spectrum. The light has changed course 135 degrees, with two refractions and one 45 degree angle. There are only two degrees where both visible sides are reflecting mirrors, and that's looking 90 degrees adjacent to and perpendicular through the hypotenuse on top. With the hypotenuse on the bottom, and the light perpendicular, it would be going down through two transparent planes. There are two crossing emitted spectrums leaving at 7 1/2 degrees out of the hypotenuse in this configuration. RIGHT ANGLE DOWN, HYPOTENUSE AT 45 DEGREES Start with the prism in a right angle down position, the other right angle vertical
facing the sun, and the hypotenuse parallel and equal to the light's angle
of incidence. Entrance refraction is 7 1/2 degree making the effective angle
37 1/2 degrees. The total reflection is 105 degrees, making the internal
exit angle 142 1/2 degrees, or 37 1/2 degrees the same as the entering
refracting angle. Subtract 7 1/2 degrees for the refraction leaving tax, and you are
left with a 30 degree emerging angle for this stream of light that's been,
intensified by reflection off an internal surface mirror, color dispersed
by the substance and spectrum united by transparent compression. These activated
parallel pulsing protons are having a ball. Light enters at 45 degrees and
exits at 30 degrees, with a spectrum. This spectrum only happens reflected
off the mirrored first half of the condensed base.
INTERNAL, POLARIZED EDGE LIT SPECTRUMS Magenta and cyan, edge polarized spectrum colors, will light up a dark line object that's not thick enough to show a dark in it's center. Diffraction's intensity merges the two edges so they touch, yellow and Ultramarine Blue are eliminated from this spectrum by diffraction. As long as there's a dark center for Ultramarine Blue to form in, Yellow will also form, there opposites. The "brighter light" image, is separate from the opaque curtain mirror reflection and is ever present over the transparency phase. Here's an example, you are looking through the prism, listening to some ol' hoot owl by the campfire. The hypotenuse is down and you are going to exercise your left eye through prisms left side. You're watching the fire and marveling over the perfect spectrum when two white dots appear from nowhere in the middle of the view, you're worried about your sanity when you hear your girlfriend coming from 7 1/2 degrees left. That's way off to your left side in the first seven and a half degrees of image. ??RIDDLE?? What were the white dots? Her headlights. The brighter light effect will override transparency and turn the hypotenuse into a mirror. The prism is a trinity and this is the third effect, the brighter light, ghost effect. There is never transparency in the mirror but there's a bright light mirror effect in the transparency. The ghost. Look through
the top position's, left transparent plane, two different opposing edge angles
light up with half the spectrum each, Yellow and Red left and Cyan and
Ultramarine
Blue right. The right side plane reverses the color stations. These four
colors are opposite, just like the real color wheel.
White light is dispersed by entering the substance, it shows up on the leading edges of two groups of edge angles in the prism, the horizontal or the vertical. With the hypotenuse on the bottom and the prism horizontal, the lit spectrum is on horizontal edges, not vertical. Turning the prism to vertical lights up the vertical edges, only. Spinning the prism 90 degrees reverses the spectrum's color order. The station change happens on the beam reach, where the radius lines are neutral and not polarized. Viewing the prism in the top, vertical, position, looking through the plane left of center and through the hypotenuse, the red and yellow half of the spectrum appear on the right side of the darker object's edges. Red touching dark and yellow merging white. There is no distortion by compression in the transparent view from this top dead center position, only refraction and dispersion. From the top vertical position, look through the inclined right plane, Ultramarine Blue is touching the right side's dark edge and Cyan fades to White. Just the opposite of the left planes stations of Red and Yellow, this is polarization. The internal prism's spectrum is two reversing half circles. This color division is "color polarizing" at 90 degrees to the length of the prism, or, at right angle's to the length of the prism. Horizontal to vertical, minimum to maximum colored light edges in 90 degrees of rotation. While the vertical position lights up the vertical edge, the horizontal radius lines or dark edges are normal looking, they are changing polarized light stations. With the polarized rotation, while vertical is in the maximum colored light phase, yellow and magenta change stations from the bottom horizontal edge to the top horizontal edge of a dark square. Starting at 1 degree with a no color unpolarized edge, to 90 degrees with a fully polarized spectrum. Any white sun spot surrounded by dark shadow will show great complete spectrums with one degree missing along prism's beam axis. It will look like two crescent moons touching points. Indoor rainbows! There is no green in the center of the transmitted pattern from a strong light prism spectrum, white light takes it's place, there is no magenta at the outsides, because red and yellow enter at the same time on the same side. Further dispersion can drop out the yellow and ult.blue of an internal spectrum by reducing the width of the edge to a line. The prisms internal spectrum color-polarizes opposite every 180 degrees of rotation.
EXTERNAL LIGHT SPECTRUM The external
projected spectrum uses the compressed two lit edges with the white in the
middle to make the projected spectrum. The infringing mirrored line of
demarcation forces the compression till there's nothing left but the spectrum colors.
In that degree, transmitted by incident light, is the projected full
spectrum.
LIGHT ENTERING A SPHERE The light
you see from a particular droplet in a rainbow has been refracted and converged
once on entering the outer convex spherical shape of the droplet. It converges
to a focus point at the center of curvature where the image inverts. The
rays are reflected and converged the second time off the concave rear and
inverted again at the focus point. The ray is than refracted and expanded
by leaving the convex lens. Some of the rays are viewed at 135 degrees, 45
degrees off center, the spectrum angle. Presto! A dispersion prism refracting
mirror. Check it out, put water in a curved bottom glass, start with the
sun behind you and find the color. It will be at the sides of the glass. SUNDOGS AND HALOS Some say, sundogs are rainbows off high atmospheric ice crystals, with the sun at the center and red on the inside. There's a darker area to the inside of these sundogs and a lighter area beyond. This is an opposite polarized rainbow. Others say a sundog is a sun ray made by clouds below the horizon line, blocking part of the sun's direct light in the upper atmosphere, the defracting long spectrum waves of the warm magenta and red bands. We're seeing diffraction in real time. Light is angling around a solid object, the earth, and heading back into the dark void left by the earth's shadow.
RAINBOWS 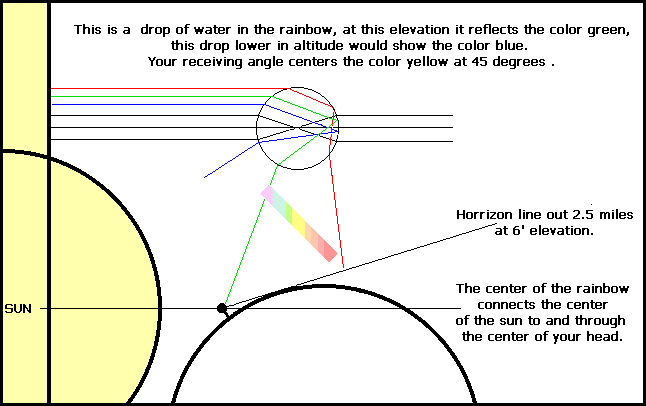
You are between the sun and the rainbow, the sun's at your back. A normal rainbow has a lighter area of sky under it, white is the reflected color under the rainbow, dark, or no added reflected light is over the top, over the red. Yellow light is in the middle and warm magenta is barely visible on top of the red. If you make your own rainbow with a small spray of water, you won't see the green, white will take it's place. Red is on the outside of the main rainbow and on the inside of the second outside rainbow, the second rainbow is separated by 15 degrees actual. It refracts light to you from the start of the other 180 degrees of polarized light, like rotating the prism. That's why it's reversed. The third rainbow is inside the main rainbow, and it's color progression is the same as the first, ad infinitum. White light is reflected in the inside area of a complete circle rainbow and under a half circle rainbow. A rainbow has depth to it, all along the radius refraction angle. A halo around the moon on clouds has red and magenta on the outside, the yellow is minor, green and cyan are the inside colors. The lighter area is to the inside. This is exactly opposite a sundog-rainbow. A moon bow is magenta, white, green, and rare. The opposite of white is clear, not black, both are without color. Black is a hypothetical dark, best seen without light. Use the main rainbow's perpendicular drop line as the protracted zero angle, the
second rainbow is at a 45 degrees closer angle to you, polarized and reversed.
This condenses the 15 degrees actual, to 7 degrees, from your viewpoint. RAINBOWS POSITION The shadow of your head is the center of the rainbow, if you ever happen upon "The Specter of Haleakala" you'll see your shadow on a valley of clouds with a rainbow around yourself. Extend this line from your heads shadow forward and back to the sun, each eye sees a different rainbow, use your right eye and call this the rainbow's "connecting center axis line". The rainbow's plane is perpendicular to this line, the circumference and width of the rainbow are relative to it's distance away from you. Have you ever seen a bright rainbow with a clear sky behind it and the rain long gone? A colloidal rainbow, the water's almost a vapor. Move the droplet a few degrees above or below the spectrum angle the angle of incidence at the back of the droplet for that color)on the rainbows plane and receive each individual wavelengths color. Find the radius path where the mirror can be moved toward you and away from you keeping the same 45 degree reflection angle to the sun. If your distance from the mirror stays constant and the reflection angle along the line of sight radius line stays constant, it will constantly reflect the sun back to you, that's the path of the rainbow, the arc of constant reflection. The second rainbow is reversed because we're seeing the condensed spectrum of the other polarized half at it's weaker end. This other spectrum angle of the droplet is also "color-polarized" by dispersion and compression. IT'S LIKE WE'RE IN THE CENTER OF A GAS CRYSTAL. From your viewing position the distance is 7 1/2 degrees between rainbows, this darker area is the axis neutral zone between the polarized halves of the atmosphere "crystal". The main rainbow's reflection angle to you is 45 degrees on the sun's center axis line. The rainbow itself is 90 degrees off the sun's center axis line. If the sun is overhead and you spray a homemade rainbow-halo around yourself, the main halo is 45 degrees below the horizon line and second halo is 15 degrees distance from the main halo, 30 degrees below the horizon line. The same ratio works for the real rainbow above the horizon line. Use the main rainbow's perpendicular drop line as the protracted zero angle, the second rainbow is at a 45 degree closer angle to you, polarized and reversed. You're viewing angle compresses the distance to 7 1/2 degrees. This is an unpolarized zone. The second rainbow is reversed and less bright because we're seeing the rainbows polarized version at it's weakest end. A rainbow moves with you, as can be seen when your plane's shadow on the clouds has a rainbow around it, or you drive in your car. One particularly
beautiful rainbow is called the "King's Rainbow" here on Maui. Because we're
on an island, the sun can be at eye level on the horizon line. The sun on
the horizon line will put the rainbow directly overhead and off to the sides
of you, 90 degrees from you to the sun. Looking to the rainbow touching the
earth it appears to go straight up, no curve, straight up. The King's
Rainbow. Note, this is just a thought.
Consider the earth's gas atmosphere as having the same properties as a crystal. As an uncompressed solid compound, still retaining the properties of a solid crystal. The Tourmaline group has all of the same properties as our air crystal.
 Order this complete color course on DVD, $35.00. 
|